Case Report - Journal of Veterinary Medicine and Allied Science (2017) Volume 1, Issue 1
The love and death relationship between lactoferrin and bacteria
Luisa Samaniego-Barrón, Christian Ávalos-Gómez and Mireya de la Garza*Departamento de Biología Celular, Centro de Investigación y de Estudios Avanzados del IPN (CINVESTAV-IPN), Avenida Instituto Politécnico Nacional No. 2508, Colonia San Pedro Zacatenco, CdMx 07360, Mexico.
- *Corresponding Author:
- Mireya de la Garza
Researcher and Professor
Department of Cell Biology
Center for Research and Advanced Studies of the National
Polytechnic Institute (CINVESTAV-IPN)
Mexico
Tel: (52-55) 5747-3987
E-mail: mireya@cell.cinvestav.mx
Accepted Date: April 15, 2017
Citation: Luisa SB, Christian AG and Mireya G. The love and death relationship between lactoferrin and bacteria.. J Vet Med Alled Sci 2017;1(1):${pages}.
Introduction
Lactoferrin (Lf) is a non-haem iron-chelating glycoprotein of 70-80 kDa that is part of the innate immune system and is present in body secretions of mammals, like bile, pancreatic juice, intestinal secretions, bronchoalveolar fluid, semen, vaginal secretions, tears, and saliva [1]. In bovine colostrum, lactoferrin is present in high concentration (1 a 2 mg/mL) and in mature milk in lower concentration (0.1-0.3 mg/mL) [2,3]; Lf is part of the protection to the newborn. In addition, Lf is produced by the secondary granules of polymorphonuclear neutrophils, from which is released following activation in infection sites.
The molecule consists of two lobes, each one with two domains, and each lobe can bind one ferric-iron ion reversibly; the apo-form is iron-free (apo-Lf) whereas the holo-form binds one or two iron ions (holo-Lf) [4]. Interestingly, Lf has multiple functions: it is microbiostatic, by chelating the iron necessary for pathogens in fluids and mucosae; it possesses serine protease activity, affecting adhesins and the secretion system type III; Lf avoids biofilm formation; it is immunomodulator by decreasing the release of some interleukins and enhancing monocyte and natural killer cell cytotoxicity; Lf has microbicidal effect against bacteria, fungi and viruses; and it has been tested in combination with antibiotics to treat some infections in humans and animals [5-7].
The Love
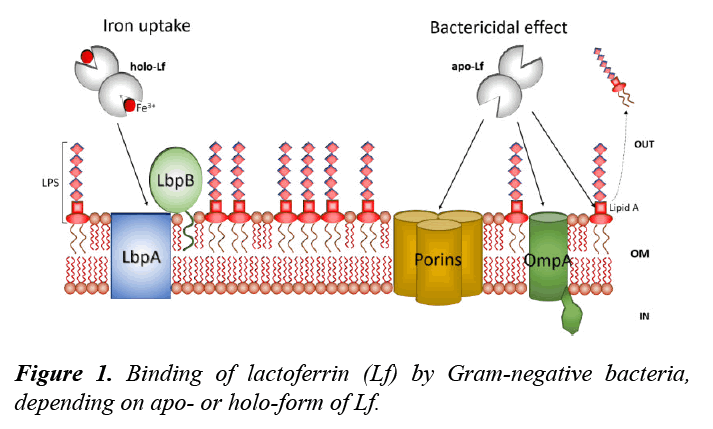
Iron is an essential micronutrient for virtually all organisms. However, it is not free inside the organism; specific systems for transport, utilization, and storage of this element have been developed by cells. Most of iron in mammals is associated to proteins, one of them is Lf [8,9]. Several pathogens are able to use holo-Lf as an iron source, such as species of the Neisseriaceae and Moraxellaceae families; for example, the veterinary pathogen Moraxella bovis [9,10]. The bacterial iron uptake is through a receptor complex, consisting in two outer membrane proteins (OMPs): a TonB-dependent integral membrane protein (LbpA) and a peripheral lipidated protein (LbpB) (Figure 1) [11-13]. LbpA is predicted to have large surface loops to bind Lf, forcing the separation of the domains surrounding the iron-binding sites to release iron, whereas LbpB is attached to the outer membrane with an N-terminal lipid anchor; it is possible that LbpB may act as initial binding site for holo-Lf [9,13]. In this case, the relationship between Lf and pathogens is beneficial to the bacteria that use this glycoprotein as an iron source for growth.
LPS: Lipopolysaccharide; Lbp: Lactoferrin binding protein; OM: Outer membrane; OmpA: Outer membrane protein A
The Death
In other cases, the binding between Lf and pathogens results in a fatal relationship. Apo-Lf can be bactericidal in certain Gramnegative species by altering the outer membrane, this is due to Lf binds to the lipid A portion of lipopolysaccharide (LPS) causing its release (Figure 1) [14]. Lf also binds to porins affecting its permeability to ions and antibiotics [15,16]. Lf is bactericidal for Gram-positive bacteria by binding to teichoic acid [7]. Our research group has demonstrated that bovine apo- Lf has bactericidal effects against the veterinary pathogens Actinobacillus pleuropneumoniae and Mannheimia haemolytica; the MIC found were 10-14 μM and 4-7 μM, respectively. Also, we have reported the binding between bovine Lf and OMPs in M. haemolytica [17,18]. Interestingly, both apo-Lf and holo-Lf were bound to two OMPs of 32.9 and 34.2 kDa with estimated IP of 8.18 and 9.35, which were identified as OmpA (heat-modifiable protein) and a membrane protein (porin), respectively [18]. In other pathogens as Escherichia coli and Salmonella enterica serovar Typhimurium the correlation of antibacterial activity of apo-Lf and binding to porins has been demonstrated [15,19]. In addition, Erdei et al. [16] evidenced the interaction of Lf with OmpF and OmpC porins.
As we describe above, the binding to Lf could be beneficial or harmful to bacteria. Holo-Lf can be used as an iron source for some pathogens, whereas apo-Lf can kill them.
References
- Pan Y, Rowney M, Guo P, et al. Biological properties of 59 lactoferrin: an overview. Aust J Dairy Technol. 2007;62(1):31-42.
- Vogel HJ. Lactoferrin, a bird’s eye view. Biochem cell Biol. 2012;90(3):233-44.
- Adlerova L, Bartoskova A, Faldyna M. Lactoferrin: A review. Vet Med Praha. 2008;53(9):457-68.
- Baker HM, Baker EN. Lactoferrin and Iron: structural and dynamic aspects of binding and release. BioMetals. 2004;17(3):209-16.
- Farnaud S, Evans RW. Lactoferrin a multifunctional protein with antimicrobial properties. Mol Immunol. 2003;40(7):395-405.
- Drago-Serrano ME. Actividades antibacterianas de lactoferrina. Enfermedades Infecc y Microbiol. 2006;26(2):58-63.
- Orsi N. The antimicrobial activity of lactoferrin: Current status and perspectives. BioMetals. 2004;17(3):189-96.
- Griffiths E. Iron in biological systems. In: Bullen JJ, Griffith E, editors. Iron and infection: molecular, physiological and clinical aspects. first. U. K.: John Wiley & Sons.
- Krewulak KD, Vogel HJ. Structural biology of bacterial iron uptake. Biochim Biophys Acta - Biomembr. 2008;1778(9):1781-804.
- Beddek AJ, Schryvers AB. The lactoferrin receptor complex in Gram negative bacteria. Biometals. 2010;23(3):377-86.
- Wong H, Schryvers AB. Bacterial lactoferrin-binding protein A binds to both domains of the human lactoferrin C-lobe. Microbiology. 2003;149(7):1729-37.
- Gray-Owen SD, Schryvers AB. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4(5):185-91.
- Yu R-H, Schryvers AB. Bacterial lactoferrin receptors: insights from characterizing the Moraxella bovis receptors. Biochem cell Biol. 2002;80(1):81-90.
- Appelmelk BJ, Geerts M, Thijs BG, et al. Lactoferrin Is a Lipid A-Binding Protein. Infect Immun. 1994;2628-32.
- Ellison RT, Giehl TJ, LaForce FM. Damage of the outer membrane of enteric gram negative bacteria by lactoferrin and transferrin. Infect Immun. 1988;56(11):2774-81.
- Erdei J, Forsgren A, Naidu AS. Lactoferrin binds 91 to porins OmpF and OmpC in Escherichia coli. Infect Immun. 1994;62(4):1236-40.
- Luna-Castro S, Aguilar-Romero F, Samaniego-Barrón L, et al. Effect of bovine apo-lactoferrin on the growth and virulence of 95 Actinobacillus pleuropneumoniae. BioMetals. 2014;27(5):891-903.
- Samaniego-Barron L, Luna-Castro S, Piña-Vázquez C, et al. Two outer membrane proteins are bovine lactoferrin-binding proteins in Mannheimia haemolytica A1. Vet Res. 2016;47(1):93.
- Naidu SS, Svensson U, Kishore AR, et al. elationship between antibacterial activity and porin binding of lactoferrin in Escherichia coli and Salmonella typhimurium. 1993(2);240-5.