Research Article - Biomedical Research (2017) Volume 28, Issue 4
The increment of mean platelet volume in early stages of prediabetes and type 2 diabetes mellitus
Huseyin Kurt1*, Davut Demirkiran1, Yasin Sari1, Omer Toprak1, Hayrettin Kara2 and Burcu Caner11Department of Internal Medicine, School of Medicine, Balikesir University, Balikesir, Turkey
2Department of Medical Biochemistry, School of Medicine, Balikesir University, Balikesir, Turkey
- *Corresponding Author:
- Huseyin Kurt
Department of Internal Medicine
School of Medicine
Balikesir University, Turkey
Accepted date: September 13, 2016
Abstract
Objective: Mean platelet volume (MPV) level is known to increase in diabetes mellitus (DM), and prediabetes. In this study the first aim was to compare MPV levels of individuals with normal glucose tolerance (NGT) and individuals with prediabetes or DM. The second aim was to identify whether the initial lower limit value of impaired fasting glucose (IFG) being 100 mg/dl or 110 mg/dl had any impact on MPV values.
Methods: Five hundred and six individuals included in the study were divided into 7 groups according to oral glucose tolerance test results and their MPV values were examined. Group 1 was the NGT group. The IFG group was divided into two groups with one group having fasting plasma glucose (FPG) of 100-109 mg/dl and the other a FPG of 110-125 mg/dl. Group 4 was the impaired glucose tolerance (IGT) group. The IFG and IGT combination group was divided into two with a FPG of 100-109 mg/dl and a FPG of 110-125 mg/dl. Finally, group seven was the DM group.
Results: MPV values of the NGT group were significantly lower compared to all other groups (p<0.001). When MPV values of all the groups except NGT were examined, no statistical difference was found (p>0.05). In IGT group a positive correlation existed between MPV and 2 h-PG (p<0.05, r=309) and between MPV and hemoglobinA1c (p<0.05, r=463).
Conclusion: Both the DM and prediabetes group had higher MPV values compared to the NGT group. Individuals with FPG values between 100-109 mg/dl have higher MPV values than NGT. If a high MPV level is detected in individuals with a FPG of lower than 110 mg/dl, OGTT should be planned in terms of the development of DM.
Keywords
Mean platelet volume, Diabetes mellitus, Oral glucose tolerance test, Prediabetes, Impaired fasting glucose, Impaired glucose tolerance
Introduction
Type 2 diabetes mellitus (DM) characterized by hyperglycemia is a worldwide, increasing, complex metabolic disorder, and associated with a twofold higher risk of cardiovascular disease [1]. DM may be diagnosed through fasting plasma glucose (FPG) or the 2-h plasma glucose (2 h PG) value after a 75 g oral glucose tolerance test (OGTT) [2]. Prediabetes is the term used for individuals with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT) and indicates an increased risk for the future development of diabetes [2]. IGT is defined as an elevated plasma glucose level (≥ 140 mg/dl, but<200 mg/dl) 2 h PG after undergoing a 75 g OGTT in individuals with FPG level <100 mg/dl. IFG is defined differently by American Diabetes Association (ADA) and World Health Organization (WHO). IFG has been defined by ADA as FPG of 100-125 mg/dl with normal 2 h PG levels (<140 mg/dl) since 2006. WHO and European Diabetes Epidemiology Group (EDEG) defined IFG as FPG of 110-125 mg/dl with normal 2 h PG levels [3].
Individuals with DM are known to have higher rates of cardiovascular disease (CVD) compared to healthy individuals. In addition, DM is risk equivalent of CVD [4]. It is known that individuals with IGT and IFG have a tendency to develop DM; the predicted cumulative 5-6 year incidence of development of type 2 diabetes for patients with either IGT or IFG is 20-34% [5]. It is estimated that nearly 60% of patients with type 2 DM had prediabetes 5 years before diagnosis [6]. IFG and IGT are linked with an increased risk of CVD and cause for early intervention [7,8]. The failure of return of 2 h PG to FPG during OGTT carries a higher risk of death from CVD and other causes compared to those whose 2 h-PG level is less than or equal to their FPG levels [9].
Platelets play an important role in the integrity of normal hemostasis. Larger platelets with higher mean platelet volume (MPV) are hemostatically more reactive and produce higher amounts of the prothrombotic factor thromboxane A2, increasing the propensity to thrombosis [10,11]. MPV is an indicator of platelet activation and increased MPV is an independent risk factor for thromboembolism [12]. Altered platelet morphology and function have been reported in patients with DM, and MPV was found to be significantly higher in diabetic patients [13,14]. They are likely to be associated with the pathological processes and increased risk of vascular disease seen in these patients [15]. Several studies have reported a positive association between MPV and FPG in IFG and in IGT [16,17]. ADA diagnostic criteria have been used for IFG and IGT in the studies conducted so far. ADA and WHO accept the values 100 mg/dl and 110 mg/dl respectively as the lower limit in the diagnosis of IFG. In this study the first aim is to compare MPV levels of individuals with normal glucose tolerance (NGT) and individuals with prediabetes or DM. This study was conducted as there were no studies examining the lower limit values separately for both IFG and IFG+IGT diagnosis. The second aim of this study is to investigate whether the initial value of lower limit of the relationship with MPV in IFG accompanied by OGTT is 100 mg/dl or 110 mg/dl.
Patients and Methods
This cross sectional study was performed at the outpatient’s clinic of Department of Internal Medicine of Balıkesir University Hospital by the permission of Balıkesir University Ethics Committee. Informed consent was obtained from all subjects who agreed to participate in the study.
Exclusion criteria were:
• Use of a drug affecting platelet function (aspirin, ticlopidine, warfarin, or heparin),
• Using of statin therapy,
• Essential hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg in-clinic and daytime ambulatory),
• Hepatic and renal diseases.
• Acute or chronic infection.
• Male patients with hemoglobin below 12 mg/dl and female patients below 11 mg/dl.
• Abnormal platelet counts (<100 and >400 × 103/μL)
• Others: Recent major surgery, smoking, chronic alcohol consumption, pregnancy and cancer.
• All individuals diagnosed with Diabetic Retinopathy (DR) and Diabetic Nephropathy (DN), and individuals with neuropathic symptoms were excluded from the study.
Eligible individuals underwent a comprehensive assessment including documentation of medical history, physical examination, and measurement of laboratory variables. Physical examination included systolic blood pressure and diastolic blood pressure measurement with subjects sitting after a 10 minute rest using sphygmomanometer. OGTT patients were divided into 7 groups (Table 1). While the study groups were formed, FPG in IFG was examined in two parts as 100-109 mg/d and 110-125 mg/dl in group 2 and 3 and also IFG and IGT combination groups in group 5 and 6. Finally, ophthalmological examinations for DR, and spot urine protein and creatine levels for DN were investigated in patients diagnosed with DM.
| Prediabetic groups | |||||||
|---|---|---|---|---|---|---|---|
| Group | NGT | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | DM |
| Number of subjects | 63 | 92 | 74 | 42 | 42 | 48 | 145 |
| FPG (mg/dl) | <100 | 100-109 | 110-125 | <100 | 100-109 | 110-125 | >126 |
| 2 h PG (mg/dl) | <140 | <140 | <140 | 140-199 | 140-199 | 140-199 | >200 |
| Age (years) | 46.8 ± 11.3 | 50.7 ± 11.3 | 55.1 ± 10.2 | 52.1 ± 14.2 | 55.8 ± 10.8 | 57.3 ± 11.7 | 56.6 ± 10.1 |
| MPV (fl) | 8.4 ± 0.7 | 9.0 ± 0.9a | 9.10 ± 1.1a | 9.2 ± 0.8a | 8.9 ± 0.8a | 9.4 ± 1.0a | 9.1 ± 0.9a |
| Total cholesterol (mg/dl) | 205.2 ± 47.3 | 200.2 ± 40.9 | 217.6 ± 41.2 | 207.8 ± 40.2 | 217.3 ± 38.1 | 219.5 ± 40.2 | 209.2 ± 35.1 |
| Triglyceride (mg/dl) | 146.3 ± 74.2 | 137.8 ± 67.1 | 156.6 ± 74.3 | 174.2 ± 93.9 | 181.1 ± 89.2 | 154.2 ± 70.2 | 158.8 ± 79.1 |
| HDL (mg/dl) | 52.1 ± 15.8 | 53.9 ± 13.7 | 53.3 ± 11.7 | 49.6 ± 9.7 | 51.5 ± 10.5 | 50.2 ± 12.3 | 50.5 ± 12.7 |
| LDL (mg/dl) | 129.2 ± 47.2 | 117.5 ± 33.2 | 135.2 ± 15.2 | 141.1 ± 28.5 | 132.4 ± 34.1 | 138.8 ± 29.2 | 137.2 ± 29.8 |
| Hb A1c (%) | 5.5 ± 0.4 | 5.6 ± 0.3 | 5.7 ± 0.3 | 5.7 ± 0.3 | 5.9 ± 0.2 | 5.8 ± 0.3 | 6.0 ± 0.4# |
| 2 h PG: 2 h Plasma Glucose; DM: Diabetes Mellitus; FPG: Fasting Plasma Glucose; MPV: Mean Platelet Volume; NGT: Normal Glucose Tolerance. Parametric values were expressed as means with SD. ap<0.05 according to NGT (by using Tukey HSD), #p<0.05 from all groups (by using Tukey HSD). | |||||||
Table 1. Demographic and laboratory characteristics of groups.
Laboratory investigation
A standard 75 g OGTT was carried out for each participant after a follow-up 12-hour fasting. Blood samples were collected at 0, 1 and 2 hour after OGTT. Venous blood samples were taken into tubes containing ethylene diamine tetraacetic acid (EDTA). Blood samples were obtained from the antecubital vein in an upright position. Measurements were completed within 30 minute using Beckman Coulter LH 780 Hematology Analyzer (Beckman Coulter, Inc. CA, USA) equipment. Blood glucose, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol measurements and urine specimen were conducted using the Beckman Coulter AU680 Analyzer (Beckman Coulter, Inc. CA, USA). HbA1c measurements were conducted using the Menarini/ARKRAY ADAMS A1c HA-8160 (Menarini Diagnostics, Firenze, Italy) equipment.
Statistical analysis
Data were expressed as mean-standard deviation. Statistical analyses were carried out using the SPSS software version 20.0 (SPSS Inc, Chicago, IL, USA). Data were tested for normal distribution using the Kolmogorov-Smirnov test. One-way analysis of variance was used to compare the clinical characteristics among the seven groups followed by the posthoc Tukey HSD test for continuous variables. The chi-square test was used to compare the categorical parameters. Pearson’s correlation coefficients were calculated to evaluate the relationships between MPV and several clinical variables (age, sex, total cholesterol, HDL cholesterol, LDL cholesterol and triglycerides). A P-value of <0.05 was considered statistically significant.
Results
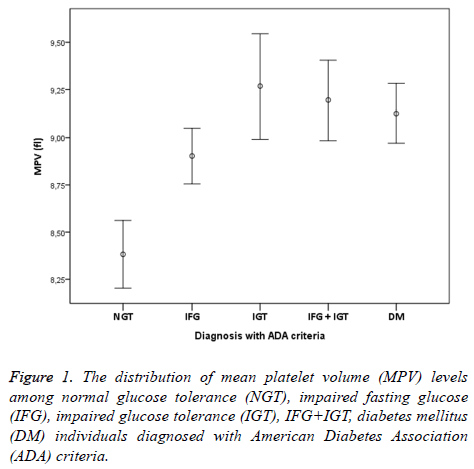
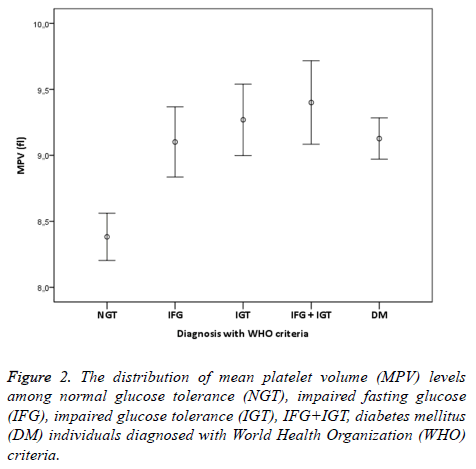
The study was conducted with 506 individuals consisting of 120 males and 386 females. The number of individuals, mean age, mean MPV and mean total cholesterol, HDL cholesterol, LDL cholesterol, triglyceride values have been shown in Table 1. The distribution of males and females in groups was similar (p>0.05). The comparison of lipid parameters, among groups did not reveal any significant difference. HbA1c values were similar in all groups but DM group (p<0.05). MPV values of the NGT group were significantly lower compared to all other groups (p<0.001). In group 2, who have normal 2 h PG values and 100-109 mg/dl FPG values, MPV values were significantly higher than group 1 individuals with NGT (p<0.001). MPV values of group 3 who fit IFG definition of WHO were significantly higher than the individuals with NGT (p<0.001). Additionally, MPV values of group 2+3 who were consistent with IFG definition of ADA were significantly higher that the individuals with NGT (p<0,001). Moreover, MPV values of group 4 fitting IGT definition were statistically higher than the individuals with NGT (p<0,001). Individuals who were consistent with the definition of both IFG and IGT were investigated separately according to ADA and WHO criteria. MPV values of group 6 who were suitable for WHO IFG+IGT combination were statistically significant compared to individuals with NGT (p<0,001).
In addition, MPV values of group 5+6 consistent with ADA IFG+IGT association were significantly higher than the individuals with NGT (p<0.001). Finally, MPV values of group 7 who were diagnosed with DM accompanied by OGTT and had no microvascular complications of diabetes were significantly higher than individuals in the control group (p<0.001). When MPV values of other groups except the individuals with NGT were examined, no statistical difference was found (p>0.05). IFG, IGT, IFG+IGT and DM diagnosis concluded whether according to ADA or WHO were both had higher MPV levels compared to NGT (Figures 1 and 2). In individuals diagnosed with IGT, positive correlation was identified between MPV and 2 h-PG values (p<0.05, r=309) and HbA1c values (p<0.05, r=463). On the other hand, in individuals diagnosed with DM, positive correlation between MPV and FPG was found (p<0.05, r=171). No correlation existed among MPV and lipids, age and gender.
Discussion
It is already known that the value of MPV is higher in patients with DM, IFG and IGT compare to NGT [16-19]. The lower limit of FPG has been used as ADA criteria in the studies conducted so far. In the current study, we found an increase in MPV levels compared to individuals with a NGT who did not fit WHO definition and had FPG levels of 100-109 mg/dl. Thus, it can be said that an increase in MPV occurs in individuals diagnosed with IFG in the early period compared to NGT. Shimodair et al. reported that MPV levels increased compared to NGT and correlation existed between MPV and FPG in individuals with IFG [20]. In addition, they stated that ADA criteria are more sensitive in the separation of prediabetic individuals from individuals with NGT than WHO and EDEG criteria, whose lower level of FPG is 110 mg/dl. However, no data were shared related to individuals having values lower or higher than 110 mg/dl [20]. Yet, in the studies conducted so far, IFG, IGT, and DM have usually been investigated separately and/or together. In our study, MPV values of individuals at all stages of glucose metabolism disorders including the initial diagnosis of prediabetes and type 2 DM, and MPV values of individuals with NGT were compared. Prediabetes consists of three sub-groups including IFG, IGT and IFG+IGT. In this study, it was found that MPV values were higher in all subgroups of prediabetes and DM compared to NGT. In the previous studies, it was discovered that MPV values increased in individuals with IFG [17,21,22]. Additionally, Çoban et al. identified an increase in MPV in individuals with IGT, which is similar to our results. [16]. It can be expected that MPV increase in combination with IFG+IGT might occur as both IFG and IGT increase. However, no study has revealed that kind of results. According to our review of literature, it can be said that our work is the first study investigating MPV levels in the IFG and IGT combination and also demonstrating an increase compared to individuals with NGT.
Hyperglycemia is known to increase atherogenicity by increasing MPV level [20,23]. During OGTT, the correlation between changes of 2 h-PG levels and MPV levels also supports this notion [10]. In the present study, in individuals diagnosed with IGT, there existed a positive correlation between MPV levels, and 2 h-PG and HbA1c levels. Although Çoban et al. claimed that there was a statistical correlation between MPV and 2 h-PG in individuals with IGT, they did not mention about the correlation related to HbA1c [16]. Demirtunc et al. found significant positive correlation between MPV and HbA1c levels in diabetic patients [24]. HbA1c level is determined both by FPG and post-prandial plasma (PPG) glucose levels. As 2 h PG remains high in individuals with IGT, it can be said that the change in 2 h PG levels is similar to change in HbA1c levels and this similarity can be associated with MPV levels. However, this similarity did not emerge in individuals diagnosed with DM. In the present study, we found a correlation between MPV and FPG in patients with type 2 DM. Hekimsoy et al. did not find any correlation between MPV and FPG but Ulutas et al. found correlation between MPV and FPG in patients with type 2 DM, Shimodaira et al. also confirmed a relationship between MPV and FPG in prediabetic subjects [15,20,25]. We did not find any correlation between MPV and FPG in prediabetic subjects. Data on the relationship between MPV and FPG in DM and prediabetes are complex and not fully explained.
Increased platelet activity has an important role in the development of vascular complications in type 2 DM [23]. Hendra et al. showed that MPV was higher in patients with DM who had a history of acute myocardial infarction (AMI) when compared with the patients with DM who did not have a history of AMI [26]. MPV can be used as a favorable test in the monitoring of type 2 DM in terms of atherosclerosis development. In addition, MPV is known to increase in individuals suffering micro-macrovascular complications of DM [15]. In the current study, from the individuals diagnosed with DM after OGTT, the ones who did not suffer from microvascular complications were included, and MPV levels of these individuals were found to be high. These findings indicate that platelet function abnormalities may occur before the development of vascular complications in diabetic patients [24]. Therefore, as soon as individuals are diagnosed with Type 2 DM atherosclerosis development should immediately be monitored. MPV increase may occur in individuals who have microvascular complications of DM such as coronary artery disease, cerebrovascular disease and peripheral artery disease [27-29]. Increase in MPV is now emerging as an independent risk factor for thromboembolism, stroke and myocardial infarction [30,31]. MPV can be used as a simple economical test in the monitoring of DM and thereby it helps curbing the morbidity and mortality. In the case of the presence of high MPV levels in individuals with NGT, caution related to prediabetes or DM is advised. In addition, if high MPV level is detected in individuals with NGT, OGTT should be planned.
The present study had several limitations. First, MPV measurements from the blood samples taken from individuals were performed immediately (less than 30 minute). However, if sodium citrate had been added to the blood taken from individuals, the platelet swelling induced by EDTA could have been avoided. We believe that interaction with EDTA was minimal as blood samples were immediately examined. Second, our findings are based only on the Turkish population; different results might be observed in other ethnic groups. As a result, MPV levels increase in DM and all stages of prediabetes compared to individuals with NGT. As MPV level increases in individuals who have FPG levels lower than 110 mg/dl, those patients should be monitored closely in terms of DM and OGTT planning should be done in the early period.
References
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 2010; 375: 2215-2222.
- Association AD. Diagnosis and classification of diabetes mellitus. Diabetes care 2015; 38: 8-16.
- Organization WH. Prevention of blindness from diabetes mellitus: World Health Organization; 2006.
- Lorber D. Importance of cardiovascular disease risk management in patients with type 2 diabetes mellitus. Diabetes, metabolic syndrome and obesity targets and therapy 2014; 7: 169-183.
- Sherwin RS, Anderson RM, Buse JB, Chin MH, Eddy D, Fradkin J. The prevention or delay of type 2 diabetes. Diabetes Care 2003; 26: 62-69.
- Irons BK, Mazzolini TA, Greene RS. Delaying the onset of type 2 diabetes mellitus in patients with prediabetes. Pharmacotherapy 2004; 24: 362-371.
- Smith NL, Barzilay JI, Shaffer D, Savage PJ, Heckbert SR, Kuller LH. Fasting and 2-hour postchallenge serum glucose measures and risk of incident cardiovascular events in the elderly: the Cardiovascular Health Study. Arc Int Med 2002; 162: 209-216.
- Ciccone MM, Scicchitano P, Cameli M, Cecere A, Cortese F, Dentamaro I. Endothelial function in pre-diabetes, diabetes and diabetic cardiomyopathy: a review. J Diabetes Metabol 2014; 5: 364.
- Ning F, Tuomilehto J, Pyorala K, Onat A, Soderberg S, Qiao Q. Cardiovascular disease mortality in Europeans in relation to fasting and 2-h plasma glucose levels within a normoglycemic range. Diabetes Care 2010; 33: 2211-2216.
- Shimodaira M, Niwa T, Nakajima K, Kobayashi M, Hanyu N, Nakayama T. Correlation between mean platelet volume and blood glucose levels after oral glucose loading in normoglycemic and prediabetic Japanese subjects. J Diabetes Invest 2014; 5: 66-71.
- Colwell JA, Nesto RW. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care 2003; 26: 2181-2188.
- Park Y, Schoene N, Harris W. Mean platelet volume as an indicator of platelet activation: methodological issues. Platelets 2002; 13: 301-306.
- Papanas N, Symeonidis G, Maltezos E, Mavridis G, Karavageli E, Vosnakidis T. Mean platelet volume in patients with type 2 diabetes mellitus. Platelets 2004; 15: 475-478.
- Jindal S, Gupta S, Gupta R, Kakkar A, Singh HV, Gupta K. Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology 2011; 16: 86-89.
- Hekimsoy Z, Payzin B, Ornek T, Kandogan G. Mean platelet volume in Type 2 diabetic patients. J Diabetes Complications 2004; 18: 173-176.
- Coban E, Kucuktag S, Basyigit S. Platelet activation in subjects with impaired glucose tolerance. Platelets 2007; 18: 591-594.
- Coban E, Bostan F, Ozdogan M. The mean platelet volume in subjects with impaired fasting glucose. Platelets 2006; 17: 67-69.
- Dindar S, Cinemre H, Sengul E, Annakkaya AN. Mean platelet volume is associated with glycaemic control and retinopathy in patients with type 2 diabetes mellitus. West Indian Med J 2013; 62: 519-523.
- Ayhan Tuzcu E, Arica S, Ilhan N, Daglioglu M, Coskun M, Ilhan O. Relationship between mean platelet volume and retinopathy in patients with type 2 diabetes mellitus. 2014; 252: 237-240.
- Shimodaira M, Niwa T, Nakajima K, Kobayashi M, Hanyu N, Nakayama T. Correlation between mean platelet volume and fasting plasma glucose levels in prediabetic and normoglycemic individuals. Cardiovascular Diabetol 2013;12: 14.
- Zuberi BF, Akhtar N, Afsar S. Comparison of mean platelet volume in patients with diabetes mellitus, impaired fasting glucose and non-diabetic subjects. Singapore Med J 2008; 49: 114-116.
- Ozder A, Eker HH. Investigation of mean platelet volume in patients with type 2 diabetes mellitus and in subjects with impaired fasting glucose: a cost-effective tool in primary health care? Int J Clin Experimental Med 2014; 7: 2292-2297.
- Kodiatte TA, Manikyam UK, Rao SB, Jagadish TM, Reddy M, Lingaiah HK. Mean platelet volume in Type 2 diabetes mellitus. J Laboratory Physicians 2012; 4: 5-9.
- Demirtunc R, Duman D, Basar M, Bilgi M, Teomete M, Garip T. The relationship between glycemic control and platelet activity in type 2 diabetes mellitus. J Diabetes Complications 2009; 23: 89-94.
- Ulutas KT, Dokuyucu R, Sefil F, Yengil E, Sumbul AT, Rizaoglu H. Evaluation of mean platelet volume in patients with type 2 diabetes mellitus and blood glucose regulation: a marker for atherosclerosis? Int J Clin Experimental Med 2014; 7: 955-961.
- Hendra TJ, Oswald GA, Yudkin JS. Increased mean platelet volume after acute myocardial infarction relates to diabetes and to cardiac failure. Diabetes Res Clin Practice 1988; 5: 63-69.
- Isik T, Ayhan E, Uyarel H, Ergelen M, Tanboga IH, Kurt M. Increased mean platelet volume associated with extent of slow coronary flow. J Cardiol 2012; 19: 355-362.
- Bath P, Algert C, Chapman N, Neal B, Group PC. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke J Cerebral Circulation 2004; 35: 622-666.
- Li S, Wang C, Zhong XW, Li HQ, Fu XQ, Ran XW. Variance of mean platelet volume in subjects with normal glucose tolerance, impaired glucose regulation and type 2 diabetic mellitus and its relationship with diabetic peripheral artery disease. Zhonghua yi xue za zhi 2012; 92: 232-235.
- Kiskac M, Zorlu M, Cakirca M, Karatoprak C, Kesgin S, Buyukaydin B. Evaluation of the relationship between serum apelin levels and vitamin D and mean platelet volume in diabetic patients. Annales d'endocrinologie 2014; 75: 200-205.
- Tavil Y, Sen N, Yazici HU, Hizal F, Abaci A, Cengel A. Mean platelet volume in patients with metabolic syndrome and its relationship with coronary artery disease. Thrombosis Res 2007; 120: 245-250.