Research Article - Biomedical Research (2018) Volume 29, Issue 2
The expression of microRNA-155 and 127 in the neonatal SD rats with acute lung injury induced by lipopolysaccharide
Ying-ying Liu1#, Shao-bing Li2#, Jin-hui Hu1#, Zhan Shi1# and Rong Wu1*
1Neonatal Medical Center, Huaian Maternity and Child Healthcare Hospital, Yangzhou University, Huaian, PR China
2Basic Medical College, Anhui Medical University, Hefei, PR China
#These authors contributed equally to this works
- *Corresponding Author:
- Rong Wu
Neonatal Medical Center
Huaian Maternity and Child Healthcare Hospital
Yangzhou University, PR China
Accepted on October 28, 2017
DOI: 10.4066/biomedicalresearch.29-17-2945
Visit for more related articles at Biomedical ResearchAbstract
Aim: This study was to observe the changes of the gene expression of microRNA-155 and 127 in the neonatal rats with Acute Lung Injury (ALI).
Methods: Eighty neonatal SD rats were randomly divided into experimental groups (intraperitoneal injection with LPS, n=60) and control (intraperitoneal injection with NS, n=20). Neonatal rats in experimental groups (LPS 1 mg, 2 mg and 3 mg group, n=20) were received corresponding dose of LPS (1, 2 and 3 mg/kg, diluted with saline to 0.2 ml), while neonatal rats in control group were injected 0.2 ml saline respectively. Then we killed rats at 1 h, 6 h, 12 h and 24 h respectively in each group. The lung general pathological changes were observed. The expression of microRNA-155 and 127 were detected by semi-quantitative reverse transcription-polymerase chain reaction. Tumor Necrosis Factor-α (TNF-α) and Interleukelin-6 (IL-6) in Bronchoalveolar Lavage Fluid (BALF) were detected by enzyme-linked immunosorbent assay. The expression of TNF-α and IL-6 protein in lung tissue were detected by western blotting.
Results: The expression of microRNA-155 was up-regulated in the tissues and BALF in a dose and timedependent manner and microRNA-127 down-regulated. The difference were statistically significant when compared with the control group (all P<0.05). The TNF-α and IL-6 level of BALF rats with ALI in LPS groups were increased compared with the control group in a dose-dependent manner (all P<0.05). A similar trend had been seen in TNF-α and IL-6 protein level of lung tissues, the difference was statistically significant.
Conclusion: The expression of microRNA-155 and 127 are associated with severity of the ALI in a dose and time-dependent manner, and they might be considered as potential biomarkers for early diagnosis of ALI.
Keywords
Acute lung injury, Lipopolysaccharide, microRNA-155, microRNA-127.
Introduction
Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS) are characterized by increased endothelial and epithelial barrier permeability [1,2]. Mortality and morbidity of acute lung injury remains high, especially in children and infants with a mortality rate in the range of 30%-40% [3].
It is vital to provide identification support for early diagnosis of ALI as a therapeutic window to target in future clinical trials. Invasive histological examinations, pulmonary vascular permeability combined with increased extravascular lung water content has been considered a quantitative diagnostic criterion of ALI/ARDS [4]. Recently, the lack of a specific biomarker for ALI is arguably one of the main obstacles in the diagnosis and successful treatment of this syndrome [5].
MicroRNAs (miRNAs) are small (~22 nt), stable RNAs that critically modulate post-transcriptional gene regulation. The miRNAs can be ubiquitously or variably expressed in different tissues and cell types and they are known to have cell type specificity [6]. At present, miRNAs have been reported as potential biomarkers for various types of disease. It was reported that miRNAs are present in human plasma in a remarkably stable form that is not prone to RNase degradation and established the measurement of tumor-derived miRNAs in serum or plasma [7]. These discoveries generated enormous enthusiasm for the potential use of miRNAs as biomarkers of neoplastic and non-neoplastic disease.
Many studies have reported that plasma miR-127 may be a candidate biomarker for early detection and diagnosis of cancer [8-10]. The miR-155 promotes endotoxin-mediated inflammation in endothelial cells and it can be released from dendritic cells and are subsequently taken up by recipient dendritic cells [11]. In summary, miR-155 and 127 are involved in inflammation and considered as potential markers. However, the expression of miR-155 and 127 of ALI is still unknown. The aim of this study was to understand the changes of the gene expression of miR-155 and 127 in neonatal rats with ALI.
Materials and Methods
Ethical approval of the study protocol
Animal experiments were conducted in accordance with the “Care and Use of Laboratory Animals” from the National Institutes of Health Guide. The study protocols were approved by the Ethics Committee of the Anhui Medical University (He’fei, China).
Animal grouping and drug injection
LPS (coli 055: B5 L2880, Sigma) was given via the intraperitoneal injection. The SD rats (weighing 20 ± 2 g, age 3 d) were provided by Anhui Medical University experimental animal center. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Anhui Medical University (permit number: 20150106). The Eighty new-born SD rats were randomly divided into LPS 1 mg, 2 mg, 3 mg groups and the control group with 20 rats in each group. Neonatal rats were received corresponding dose of LPS (1 mg/kg, 2 mg/kg and 3 mg/kg) by intraperitoneal injection (diluted with saline to 0.2 ml) in experimental groups, while neonatal rats was injected 0.2 ml saline in control group.
Indexes and sample collection
We observed the general situation of neonatal rats in 24 h. Furthermore, we killed rats at 1 h, 6 h, 12 h and 24 h after anesthesia (2% isoflurane inhalation) employed. The fasting tissue and Bronchoalveolar Lavage Fluid (BALF) samples from the subjects were collected in containing vacutainers, and transferred into a microtube. The samples were centrifuged at 1,000 Xg and 4°C for 10 min. Tissue and BALF sample was collected carefully and aliquoted in RNase-free tubes and stored at-80 until future use. Finally, we figured out the lung general pathological changes. The gene expression of miR-155 and 127 in lung tissue and BALF were determined by semiquantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR). Tumor Necrosis Factor-α (TNF-α) and Interleukelin-6 (IL-6) level of BALF were detected by Enzyme-linked immunosorbent assay (ELISA). The expression of TNF-α and IL-6 protein in lung tissue were analysed by Western blotting.
RNA extraction and cDNA synthesis
Do not use more than 30 mg tissue. Disrupt the tissue samples and homogenize the lysate in the appropriate volume of Buffer RLT. Centrifuge the lysate for 3 min at maximum speed. Carefully remove the supernatant by pipetting. Then, tissue sample was transferred to a new microtube and total RNAs were isolated from all specimens using RNeasy® Mini Kit (Qiagen, Germany) based on the manufacturer’s instructions. The methods of total RNAs in BALF were isolated using miRNeasy Serum/Plasma Kit (Qiagen, Germany) according to the manufacturer’s instructions.
Single-stranded cDNA was prepared in a reverse-transcription reaction using TaqMan MicroRNA Reverse Transcription Kit (Thermo Fisher, American) using 5 μg of RNA, according to the manufacturer's protocol. Reverse transcription primer, miR-155: 5’- CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGC CCCTATC-3’; miR-127: 5’- AGAGGCGGAVGGTGTCGTAGTTGAAGTGAGCCGTCC- 3’; U6: oligo dT.
The transcription process included incubation of the reaction mixture at 25°C for 10 min, 42°C for 60 min, followed by 10 min at 70°C. The cDNA was stored at -80°C until further use for PCR.
Polymerase chain reaction
Primer pairs for the amplification of cDNA coding for miR-155 and miR-127 were designed by Takara Biomedical Technology Corporation (Beijing). The sequences of primers used in the experiments are presented in Table 1. PCR were carried out using cDNA, mgcl2 (10 mM), Taq-polymerase (5 U/μl), PCR buffer, dNTP (10 mM) and a pair of specific primer (10 μM) in a final volume of 20 μl each tube.
| Gene | Primers sequence | Product size (bp) |
|---|---|---|
| miR-155 F | 5'- TGCCTCCAACTGACTCCTAC -3' | 63 bp |
| miR-155 R | 5'- GCGAGCACAGAATAATACGAC -3' | |
| miR-127 F | 5'- CGGTGTCGTAGTTGAAGTGAG -3' | 42 bp |
| miR-127 R | 5'- GATGTCGGATCCGTCTGAGC -3' | |
| U6 F | 5' - CTCGCTTCGGCAGCACA - 3' | 94 bp |
| U6 R | 5' - AACGCTTCACGAATTTGCGT - 3' |
Table 1. Primer sequences.
The PCR conditions were as follows: initial denaturation at 95°C for 5 min followed by 40 cycles, annealing at 95°C for 15 s, and extension at 60°C for 20 s and final extension at 72°C for 40 s. The expected length for PCR products were shown in Table 1. PCR for each sample was duplicate. U6 small nuclear 6 (snRNA RNU6B, referred to as “U6”) was analysed as an internal control.
Gel electrophoresis
The PCR products of each interested gene and U6 were loaded on to the same ethidium bromide-stained agarose gels (1%). A 1-kb DNA ladder molecular weight marker was run on each gel to confirm expected molecular weight of the amplification product. Stained gels were recorded and the band intensity was evaluated using the Tanon 1600 software. Band intensity was expressed as relative absorbance units. The ratio between the sample RNA to be determined and U6 was calculated to normalize for initial variations in sample concentration and as a control for reaction efficiency. Mean and standard deviation of all experiments performed were calculated after normalization to U6.
The ELISA assay
IL-6 and TNF-α level in BALF were measured by enzymelinked immunosorbent assay kit (Beijing Tiantan Biological Products co., LTD) according to manufacturer’s instructions as previous research [12].
Western blot analysis
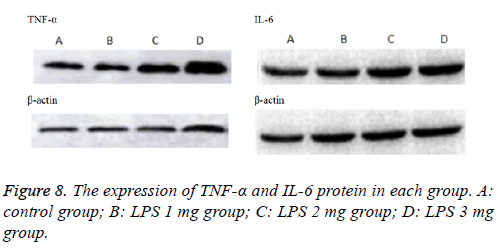
Lung tissues were harvested and homogenized using a homogenizer and tissue lysis/extraction reagent containing a protease inhibitor cocktail (both from Sigma-Aldrich). Protein concentrations were determined using a Bradford reagent (Bio- Rad Laboratories, Inc.). Western blotting was performed as previously described [13], and the following primary antibodies and dilutions used were: anti-TNF-α (1:1,000 dilution; Beyotime Institute of Biotechnology, China), anti- IL-6 (1:1,000 dilution; Beyotime Institute of Biotechnology, China) and anti-β-actin (1:1,000 dilution; Beyotime Institute of Biotechnology, China).
Histopathological lung examination
After collecting the BALF samples, lung tissue was fixed in 10% (v/v) neutral-buffered formalin. Lung tissues were embedded in paraffin, cut into 4-μm sections were visualized by light microscopy (400X) (Olympus, DP73), and stained with hematoxylin and eosin (H and E) solution (both from Sigma-Aldrich; hematoxylin, MHS-16; eosin, HT110-1-32) to estimate inflammation.
Statistical analysis
Statistical analyses were performed using the SPSS software version 17.0, and all variables were expressed as mean ± Standard Deviation (SD). Statistical significance between groups was analysed by the ANOVA followed by Tukey Post Hoc test when the variables between groups were normally distributed. The ManneWhitney U test and Kruskale Wallis test were used for nonparametric values. A p value less than 0.05 was considered statistically significant.
Results
The expression of miR-155 in neonatal rats of LPSinduced acute lung injury
To figure out the change expression of miR-155 of acute lung injury in vivo, we administered LPS into neonatal rats through intratracheal injection using established protocols [14].
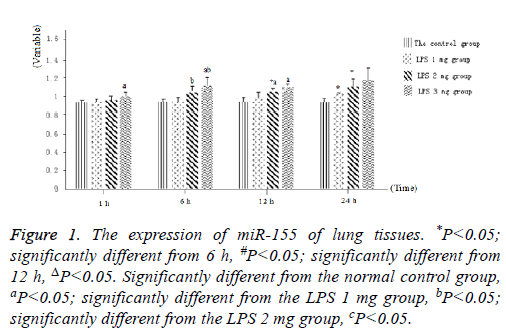
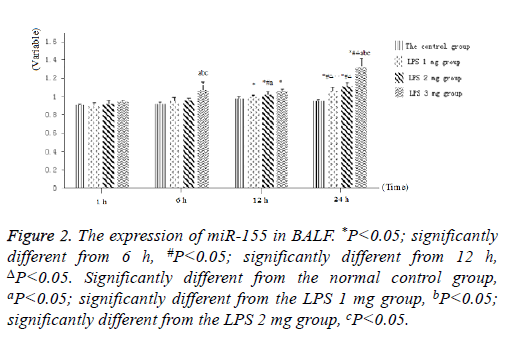
Littermate neonatal rats were used as controls. At 1, 6, 12 and 24 h after injection, lung tissues and BALF were evaluated to determine the expression of miR-155. As shown in Figure 1, as well as Figure 2 into BALF-the miR-155 level of acute lung injury-were significantly increased after LPS stimulation in LPS groups (Figure 3). These results suggested that the expression of miR-155 increase with extension of time after injection of LPS.
Figure 1: The expression of miR-155 of lung tissues. *P<0.05; significantly different from 6 h, #P<0.05; significantly different from 12 h, ΔP<0.05. Significantly different from the normal control group, aP<0.05; significantly different from the LPS 1 mg group, bP<0.05; significantly different from the LPS 2 mg group, cP<0.05.
Figure 2: The expression of miR-155 in BALF. *P<0.05; significantly different from 6 h, #P<0.05; significantly different from 12 h, ΔP<0.05. Significantly different from the normal control group, aP<0.05; significantly different from the LPS 1 mg group, bP<0.05; significantly different from the LPS 2 mg group, cP<0.05.
The expression of miR-127 in neonatal rats of LPSinduced acute lung injury
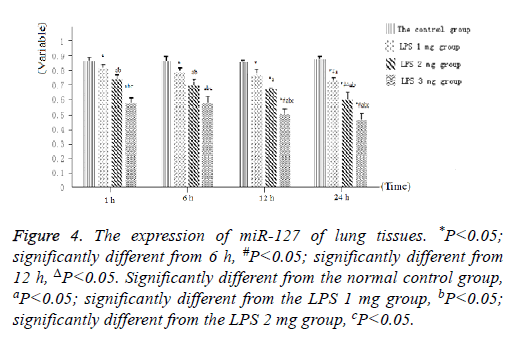
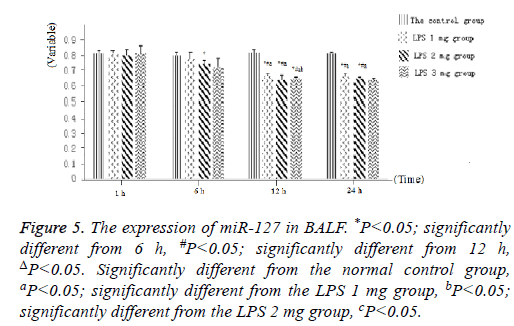
As displayed in Figure 4, as well as Figure 5 into BALF-the miR-127 level of acute lung injury-were significantly decreased after LPS stimulation in LPS groups. Compared with the control group, miR-127 expression of lung tissue and BALF in the LPS groups (normalized to u6 expression) was significantly decreased with extension of time after injection of LPS (Figure 6). There is statistical significance between the LPS groups and the control group or among LPS groups comparison (P<0.05).
Figure 4: The expression of miR-127 of lung tissues. *P<0.05; significantly different from 6 h, #P<0.05; significantly different from 12 h, ΔP<0.05. Significantly different from the normal control group, aP<0.05; significantly different from the LPS 1 mg group, bP<0.05; significantly different from the LPS 2 mg group, cP<0.05.
Figure 5: The expression of miR-127 in BALF. *P<0.05; significantly different from 6 h, #P<0.05; significantly different from 12 h, ΔP<0.05. Significantly different from the normal control group, aP<0.05; significantly different from the LPS 1 mg group, bP<0.05; significantly different from the LPS 2 mg group, cP<0.05.
Pro-inflammatory cytokine production in LPSstimulated acute lung injury
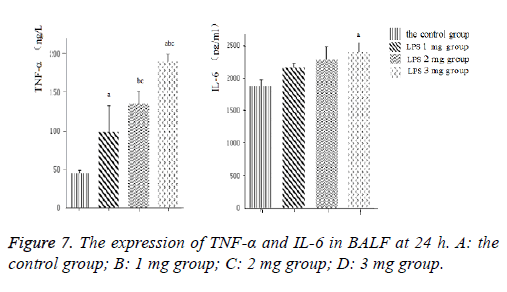
Inflammatory cytokines TNF-α and IL-6 (Figure 7) levels in the BALF increased with LPS stimulation. Induction of ALI with LPS resulted in a significant increase in TNF-α production compared with the control group, moreover, IL-6 significantly increase by LPS induction in a dose-dependent manner, the difference was statistically significant (P<0.05).Consistently, inflammatory cytokines TNF-α and IL-6 (Figure 8) protein levels production in lung tissues were all increased in neonatal rats. The more doses of LPS, the higher TNF-α and IL-6 level, the difference was statistically significant (P<0.05).
Histological examinations of lung tissue
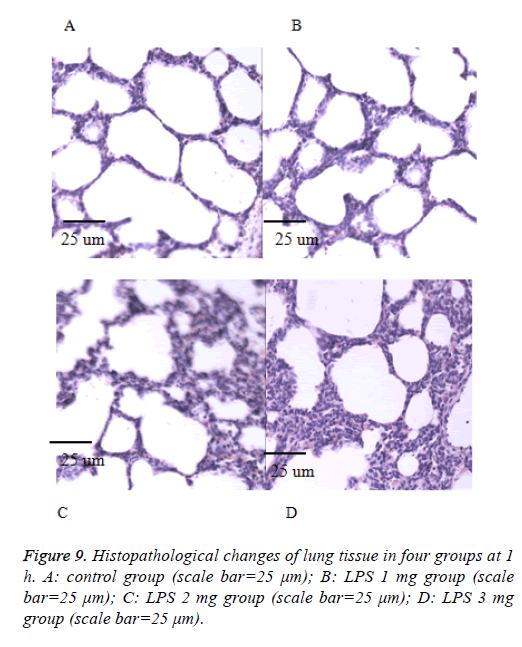
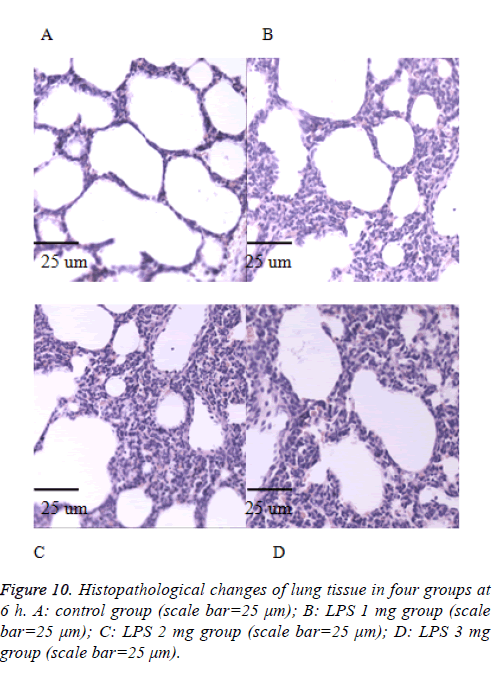
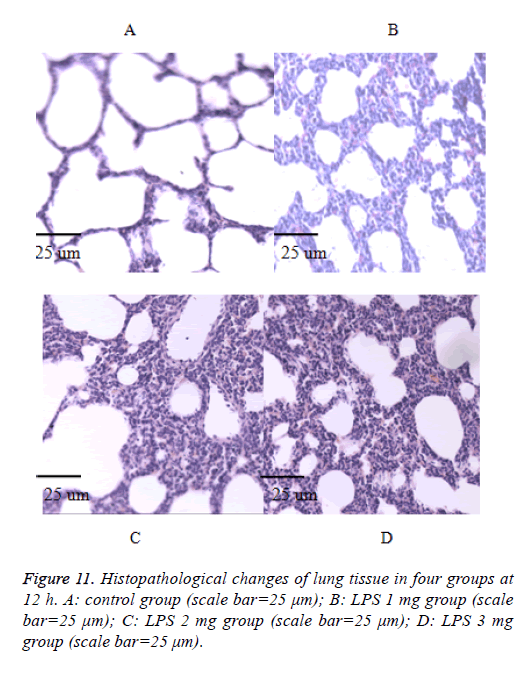
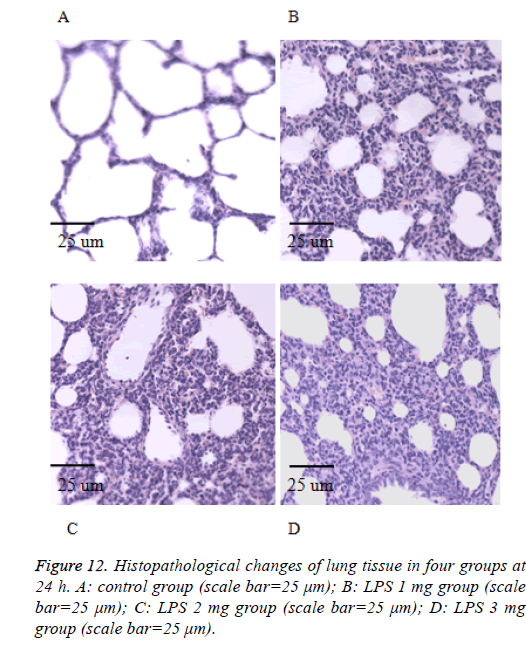
We also performed the Hematoxylin and eosin stain to examine lung pathological changes. As shown in Figures 9-12, LPSinduced lung injury was promoted in experiment groups, as manifested by increase infiltration of neutrophils, increased thickening of interstitial alveolar regions, and minimal structural damage compared with that seen in the control group. Our previous studies confirmed that all samples underwent histological evaluation, and neonatal rats of ALI were identified [14].
Discussion
ARDS may co-occur with the ALI, with significant impact on morbidity and mortality and worse prognosis. The reason is the lack of early and specific diagnostic criteria. Therefore, it is particularly vital to elucidate the pathogenesis of neonatal ALI, especially for discovering the early diagnosis of neonatal ALI. The research of miRNA and the pathogenesis, diagnosis, treatment and prognosis of ALI has been brought to researchers’ attention.
MiRNA can exist stably in the blood, body fluid, cell and tissues. A study proved that circulating miRNAs have begun to be demonstrated as highly stable, blood-based biomarkers for diseases [15]. It is reported that miR-155 were induced in the human monocytic cell line THP-1 by LPS [16]. The reports showed the up-regulation of the miR-155 in response to LPS [17], whereas two recent reports suggested an impaired response of B cells from miR-155-/- knockout mice toward LPS [18,19]. The above studies detected miR-155 level in the cellular, it’s expression in lung tissue and BALF of ALI has not been reported. Our results showed that the expression of miR-155 in lung tissue and BALF were increased gradually with the increase of dose and the passage of time in the LPS groups.
In this study, the lung tissue of experimental group showed intense perivascular, peribronchial and septal inflammatory infiltration, with a predominance of polymorphonuclear cells. The septal thickening, irregular distribution of air spaces and focal areas of alveolar hemorrhage were also observed. Its severity was increased as dose increases and time goes which was consistent with our previous studies [14]. The study have shown that miR-155 downregulated SOCS-1 expression by binding to the 3'-UTR of the SOCS-1 mRNA , and it can promote the inflammatory response during lung injury as a proinflammatory factor [20]. Previous study has indicated that miR-155 may enhance inflammatory response by stimulating the release of inflammatory mediators by targeting several negative feedback molecules, including SH2 domaincontaining inositol 5’-phosphatase (SHIP)-1 [21]. These results suggested the miR-155 maybe promote inflammatory response of LPS-induced ALI by downregulating SOCS-1 and targeting (SHIP)-1.
The present study indicated that miR-127 was prominently induced in the inflammation-related pulmonary disorders such as LPS, bleomycin, or immunocomplex-induced lung injury and inflammation [22,23], which suggesting a potential role of miR-127 in the inflammatory signaling and lung pathology. Our results showed that the expression of miR-127 in lung tissue BALF were decreased gradually with the increase of dose and the passage of time in the LPS groups. These results suggested the miR-127 may be inhibits inflammatory response of LPS-induced ALI by targeting IgG Fcγ receptor. However, another study demonstrated miR -127 promotes lung inflammation and injury by activating the JNK pathway. The mechanism of miR-127 in LPS-induced ALI should be further verified.
The study found that there was significant increase in miR-155 in the 264.7 macrophages and in wild-type C57BL/6 mice and a decrease in miR-127 in the mouse macrophage, alveolar macrophage and human monocyte [24], expression trend similar to our studies. These results indicated that miRNA-155 and miRNA-127 likely play a central role in the regulation of the response to a large panel of bacterial and viral infection in both mouse and human, which might making them to become a potential biomarkers and therapeutic targets.
Conclusion
In summary, in neonatal rats with ALI, the expression of miRNA-155 was increased in a dose and time-dependent manner, and the expression of miRNA-127 was decreased in a dose and time-dependent manner. These results suggested that the expression of miRNA-155 and 127 are associated with severity of the ALI in a dose and time-dependent manner, and they might be considered as potential biomarkers for early diagnosis of ALI.
Acknowledgements
This research was funded by grants of Key Child Health Personnel in Jiangsu Province (Project no: FRC201211).
References
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149: 818-824.
- ForceRanieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307: 2526-2533.
- Villar J, Blanco J, Kacmarek RM. Current incidence and outcome of the acute respiratory distress syndrome. Curr Opin Crit Care 2016; 22: 1-6.
- Kushimoto S, Taira Y, Kitazawa Y, Okuchi K, Sakamoto T, Ishikura H, Endo T, Yamanouchi S, Tagami T, Yamaguchi J, Yoshikawa K, Sugita M, Kase Y, Kanemura T, Takahashi H, Kuroki Y, Izumino H, Rinka H, Seo R, Takatori M, Kaneko T, Nakamura T, Irahara T, Saito N. Pulmonary Edema Study Group. The clinical usefulness of extravascular lung water and pulmonary vascular permeability index to diagnose and characterize pulmonary edema: a prospective multicenter study on the quantitative differential diagnostic definition for acute lung injury/acute respiratory distress syndrome. Crit Care 2012; 16: 232.
- Villar J, Slutsky AS. GOLDEN anniversary of the acute respiratory distress syndrome: still much work to do. Curr Opin Crit Care 2017; 23: 4-9.
- McCall MN, Kent OA, Yu J, Fox-Talbot K, Zaiman AL. MicroRNA profiling of diverse endothelial cell types. BMC Med Genomics 2011; 4: 78.
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, OBriant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008; 105: 10513-10518.
- You W, Wang Y, Zheng J. Plasma miR-127 and miR-218 might serve as potential biomarkers for cervical cancer. Reprod Sci 2015; 22: 1037-1041.
- Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell 2006; 9: 435-443.
- Lu M, Ju S, Shen X, Wang X, Jing R, Yang C, Chu H, Cong H. Combined detection of plasma miR-127-3p and HE4 improves the diagnostic efficacy of breast cancer. Cancer Biomark 2017; 18: 143-148.
- Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, OConnell RM. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun 2015; 6: 7321.
- Peng Y, Li LJ. TNF-a-308G/A polymorphism associated with TNF-a protein expression in patients with diabetic nephropathy. Int J Clin Exp Pathol 2015; 8: 3127-3131.
- Niu Y, Mo D, Qin L, Wang C, Li A, Zhao X, Wang X, Xiao S, Wang Q, Xie Y, He Z, Cong P, Chen Y. Lipopolysaccharide-induced miR-1224 negatively regulates tumour necrosis factor-a gene expression by modulating Sp1. Immunology 2011; 133: 8-20.
- Liu YY, Li SB, Hu JH, Wu R. Establishment of acute lung injury model in neonatal SD rats. Biomedical Research 2016; 27: 1245-1250.
- Mi S, Zhang J, Zhang W, Huang RS. Circulating microRNAs as biomarkers for inflammatory diseases. Microrna 2013; 2: 63-71.
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006; 103: 12481-12486.
- OConnell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc Natl Acad Sci USA 2007; 104: 1604-1609.
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science 2007; 316: 608-611.
- Thai TH., Calado DP , Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science 2007; 316: 604-608.
- Wang W, Liu Z, Su J, Chen WS, Wang XW, Bai SX, Zhang JZ, Yu SQ. Macrophage micro-RNA-155 promotes lipopolysaccharide-induced acute lung injury in mice and rats. Am J Physiol Lung Cell Mol Physiol 2016; 311: 494-506.
- Mashima R. Physiological roles of miR-155. Immunology 2015; 145: 323-333.
- Ying H, Kang Y, Zhang H, Zhao D, Xia J. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J Immunol 2015; 194: 1239-1251.
- Xie T, Liang J, Liu N, Wang Q, Li Y. MicroRNA-127 inhibits lung inflammation by targeting IgG Fcγ receptor I. J Immunol 2012; 188: 2437-2444.
- Tili E, Michaille JJ, Cimino A, Costinean S, Dumitru CD, Adair B, Fabbri M, Alder H, Liu CG, Calin GA, Croce CM. Modulation of miR-155 and miR-125b levels following lipopolysaccharide/TNF-alpha stimulation and their possible roles in regulating the response to endotoxin shock. J Immunol 2007; 179: 5082-5089.