Research Article - Biomedical Research (2017) Volume 28, Issue 21
The expression and clinical significance of Treg cells in chronic myelocytic leukemia
Yi Cheng1#, GangOu Yang2#, Mengyuan Sun1, Kai Chang1, Renjun Long1 and Zhongyong Jiang1*
1Department of Clinical Laboratory, Chengdu Military General Hospital, Chengdu Military District, Chengdu, People’s Republic of China
2Department of Respiration, Chengdu Military General Hospital, Chengdu Military District, Chengdu, People’s Republic of China
#These authors contributed equally
- *Corresponding Author:
- Zhongyong Jiang
Department of Clinical Laboratory
Chengdu Military General Hospital
Chengdu Military District, Chengdu, People’s Republic of China
Accepted on September 06, 2017
Abstract
Regulatory T cell (Treg) can regulate auto-immune response. It counteracts against autogenic or allogenic tumor antigens, and maintains immune tolerance and cell homeostasis via inhibiting various physiological or pathological immune responses. Study has found that Treg cells could inhibit the function of immune effector cells with anti-tumor activity, and played important roles in the immune escape of tumor cells. This study analyzed the expression of Treg in Chronic Myelocytic Leukemia (CML), to discuss their effects on disease treatment efficacy as well as prognosis. A total of 107 CML patients were recruited. Flow cytometry was used to measure the peripheral level of CD4+CD25+Foxp3+ Treg. Using averaged antigen expression level as the cut-off line, patients were divided into high and low level groups, whose treatment efficacy and drug resistance were evaluated. Kaplan-Meier approach was used to plot patient survival curve for comparison of survival rates. CD4+CD25+Foxp3+ Treg was significantly higher in chronic untreated group 6.22 ± 1.88% and accelerative/excess blast group 6.79 ± 2.05% compared with control group 1.81 ± 0.84% or chronic treatment group 2.06 ± 1.13%, p<0.05 in all cases). Patients with high Treg levels had lower levels of 3mCHR (3-month complete hematological remission), 12mCCR (12-month complete cytogenetic remission) and 18mCMR (18-month complete molecular remission), but higher drug resistance level than patients with low Treg levels (p<0.05). Overall survival rate of high-Treg patients was remarkably lower than that in low-Treg group (χ2=4.569, p=0.035). Peripheral level of CD4+CD25+Foxp3+ Treg can affect CML course and prognosis, and has implications for the diagnosis and treatment.
Keywords
Regulatory T cells, Chronic myelocytic leukemia, Tumor immunology.
Introduction
Chemo and radio-therapy can treat leukemia, with certain adverse effects and higher recurrent rate after treatment [1]. Such disease recurrence stems from residual tumor cells inside patients, namely Minimal Residual Disease (MRD) of leukemia. During MRD stage, patients have little tumor loading inside the body. Those leukemia cells surviving from long-term radio- or chemo-therapy had lower sensitivity to treatments, thus affecting efficacy and becoming one of reason for recurrence [2,3]. The occurrence of MRD in leukemia is correlated with the existence of body immune tolerance mechanism. CD4+CD25+Foxp3+ regulatory T cell (Treg) has dual functions in the induction of immune tolerance and immune suppression, and has been demonstrated to be related with immune escape of multiple tumor cells [4]. Beyer et al. found significantly higher peripheral level of CD4+CD25+Foxp3+ Treg in chronic lymphatic leukemia and acute myeloid leukemia patients, as well as the relationship with patient disease period and prognosis, suggesting CD4+CD25+Foxp3+ Treg as one of the important reasons for drug resistance and recurrence of hematological diseases [5,6].
Chronic Myelocytic Leukemia (CML) is a malignant clonal hematological disease featured as chronic proliferation of myelocytic cells. It can inhibit normal hematological processes of bone marrow, causing anemia, bleeding, infection and organ infiltration. In clinic, CML can be divided into chronic phase, accelerative phase, and excess blast phase [7,8]. Etiology of CML has not been fully understood, although most clinicians agreed that Philadelphia chromosome (Ph) and Bcr/Abl fusion gene were closely associated with CML [9]. Allograft hematological stem cell transplantation is currently the most effective way for the treatment of CML. Due to age and tissue matching problems, however, there was a high recurrent rate. Classical treatment approach such as α-interferon or cytosine arabinoside had unfavorable treatment efficacy with more adverse effects. Tyrosine kinase inhibitor such as nilotinib works as a molecular targeted drug, but may cause drug resistance, and was ineffective in Bcr/Abl mutation patients. Therefore, establishment of effective treatment to elongate survival period of CML patients is one major challenge currently.
This study measured the expression of CD4+CD25+Foxp3+ Treg in peripheral blood of CML patients, and investigated the effect of CD4+CD25+Foxp3+ Treg on the treatment efficacy and prognosis of CML patients, aiming to provide evidences for the diagnosis and treatment of CML.
Materials and Methods
Clinical information
A total of 107 CML patients who were diagnosed and treated in Chengdu Military General Hospital between January 2011 and June 2014 were recruited in this study. There were 56 males and 51 females, aged between 48 and 74 y old (average=50.7 ± 8.6 y). There were 56 cases of chronic patients at primary diagnosis, 31 chronic patients with treatment (by hydroxycarbamide, α-interferon or HA chemotherapy), and 20 cases in excess or blast phase. A total of 94 patients were Ph (Philadelphia chromosome) positive. All patients were diagnosed as CML by clinical observations, bone marrow biopsy, cytogenetics and molecular biology examinations based on the WHO guideline [10]. Another cohort of 60 healthy individuals was selected as the control group. There were 33 males and 27 females in this group, aged between 33 and 59 y (average age=46.8 ± 10.5 y). No significantly difference of age or sex existed between CML patients and control group (p>0.05).
This study has been pre-approved by the ethical committee of Chengdu Military General Hospital. All subjects have signed the consent forms before recruitment in this study.
Treg assay
Fasted venous blood samples (2~4 mL) were collected in the morning and kept in 4°C fridge. Flow cytometry was used to test the level of CD4+CD25+Foxp3+ Treg sub-population. In brief, 15 μL peripheral blood was kept in test tube, followed by addition of FITC-CD4, PE-CD25 and APC-Foxp3 mouse anti-human antibody (BD Pharmaingen, US; 1:1000 dilution).
Isotype antibodies were added to the samples from control group for compensation. After dark incubation for 15 min at room temperature, erythrocyte lysis buffer was added for 10 min room temperature incubation.
After centrifugation, supernatant was discarded and cell pellets were washed with PBS and re-suspended in 0.5 mL PBS followed by flow cytometry analysis of Treg cells in the suspensions using fluorescent-labelled antibodies (FITC-CD4, PE-CD25 and APC-Foxp3).
Data was analyzed using BD CellQuest Pro software (version 5.1).
Monitor of prognostic parameters
3-month complete hematological remission (3mCHR) was defined as leukocyte count<10X109/L; platelet<450X109/L; No disease symptom or body sign; No observable spleen swelling. 12-month complete cytogenetic remission (12mCCR) was defined as Ph+=0%. 18-month complete molecular remission (18mCMR) was defined as negative results for nested RT-PCR analysis of Bcr-Abl mRNA expression.
Drug resistance was classified as primary and secondary subtypes. Primary drug resistance was further assigned as primary hematological drug resistance (no CHR between 3 and 6 months) and primary cytogenetic drug resistance (No cytogenetic response within 6 month, or no CMR at 12 month, or no CCR at 18 month). Secondary drug resistance refers to the loss of hematological/cytogenetic response or disease progression.
Statistical analysis
SPSS18.0 was used to process all data analysis. Measurement data were presented as mean ± standard deviation (SD). One-way ANOVA was used for comparing means among multiple groups, followed by paired comparison in LSD test. Enumeration data was presented as percentage and tested by chi-square analysis. Kaplan-Meier method was used for plotting patient survival curve. A statistical significance was defined when p<0.05.
Results
Comparison of general information
No significant differences were observed in age or sex between patients and healthy control people (p>0.05, Table 1).
| Sex | Age | F | P | |||
|---|---|---|---|---|---|---|
| M/F | χ2 | P | ||||
| Control | 33/27 | 3.346 | 0.341 | 46.8 ± 10.5 | 1.398 | 0.245 |
| Chronic untreated | 25/31 | 48.3 ± 9.4 | ||||
| Chronic treated | 43059 | 51.2 ± 10.8 | ||||
| Accelerative blast | 42989 | 49.7 ± 9.9 | ||||
Table 1. General information of patients and control people.
Levels of CD4+CD25+Foxp3+ Treg in CML patients at different phases
The percentage of CD4+CD25+Foxp3+ Treg in all CD4+ T cells at different phases of CML was shown in Table 2. The ratio of CD4+CD25+Foxp3+ Treg in chronic untreated group 6.22 ± 1.88% and accelerative blast group 6.79 ± 2.05% was significantly higher than that in healthy control group 1.81 ± 0.84% or chronic treated group 2.06% ± 1.13%. However, no significant difference of CD4+CD25+Foxp3+ Treg existed between chronic treated and control groups (p>0.05), neither did those between chronic untreated and accelerative blast groups (p>0.05).
| N | CD4+CD25+ Foxp3+T cell (%) | |
|---|---|---|
| Healthy control | 60 | 1.81 ± 0.84 |
| Chronic untreated | 56 | 6.22 ± 1.88*# |
| Chronic treated | 31 | 2.06 ± 1.13 |
| Accelerative blast | 20 | 6.79 ± 2.05*# |
Note: *p<0.05 compared to healthy control group; #p<0.05 compared to chronic treated group.
Table 2. Correlation between CML disease phase and CD4+CD25+Foxp3+ Treg, CD4+T cells.
Effect of CD4+CD25+Foxp3+ Treg on prognosis of CML patients
Using averaged CD4+CD25+Foxp3+ Treg in all CD4+ T cells of all patients as the cut-off line, 56 patients from chronic untreated group were divided into high level (N=25) and low level (N=31) sub-groups to investigate the effect of CD4+CD25+Foxp3+ Treg on the prognosis of CML patients. As shown in Table 3, 3mCHR, 12mCCR and 18mCMR in high level group were all lower than those in low-level group. However, the incidence of drug resistance was higher in high-level group (p<0.05).
| 3mCHR (n, %) | 12mCCR (n, %) | 18mCMR (n, %) | Drug resistance (n, %) | |
|---|---|---|---|---|
| High Treg level | 16 (64.00) | 12 (48.00) | 7 (28.00) | 14 (56.00) |
| Low Treg level | 28 (90.32) | 24 (77.42) | 17 (54.83) | 9 (29.03) |
| χ2 | 5.695 | 5.217 | 4.071 | 4.159 |
| P | 0.017 | 0.022 | 0.0436 | 0.0414 |
Note: 3mCHR (3-month complete hematological remission), 12mCCR (12-month complete cytogenetic remission) and 18mCMR (18-month complete molecular remission).
Table 3. Effect of CD4+CD25+Foxp3+ Treg on prognosis of CML patients.
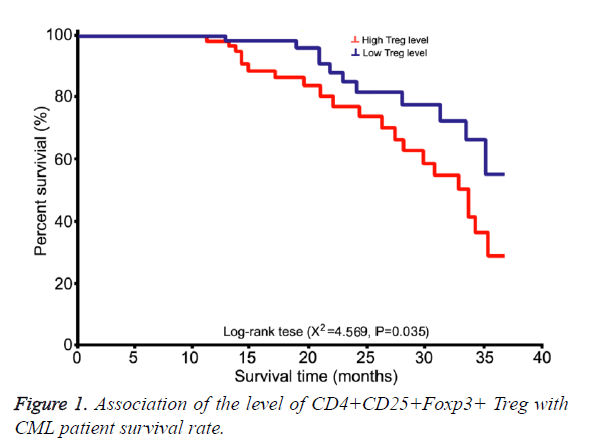
Effects of CD4+CD25+Foxp3+ Treg on CML patient survival rate
Long-term follow up (13~48 month) was performed on all 56 patients at chronic untreated group. Using averaged CD4+CD25+Foxp3+ Treg in all CD4+ T cells of all patients as the cut-off line, patients were divided into high level and low level sub-groups for survival analysis (Figure 1).
Overall survival rate in patients from high-level group was significantly lower than that from low-level group (χ2=4.569, p=0.035).
Discussion
CML is a malignant blood disorder caused by abnormal clonal proliferation of bone marrow hematological stem cells. It is featured as Ph chromosome and Bcr/Abl fusion gene [11]. CML occupies about 20% of all adult leukemia, with the median onset disease at 50~60 y. Bcr-Abl fusion gene is formed by t (9; 22) (q34; q11) gene translocation, and can induce abnormal clonal proliferation of myeloid cells via coding fusion protein and activating a cascade of signal transduction pathways [12]. In this study, the average age of patients was 50.7 ± 8.6 y (48~74 y). Ph chromosome existed in a total of 94 patients, consistent with previous reports. CML can be divided into chronic stage, accelerative stage and blast stage. During different disease phase, clinical manifestation, biological activity of tumor cells, treatment approach and prognosis were all different. During initial treatment phase, it is commonly to use hydroxycarbamide, interferon or cytosine arabinoside, all of which, however, had unsatisfactory treatment efficacy with adverse effects. Allogenic hematological stem cell transplantation is the most promising way to treat CML. However, due to limitation of age or tissue matching, it has a high mortality and cannot totally improve the treatment efficacy and long-term survival of CML. Tyrosine Kinase Inhibitor (TKI) is a target drug for Bcr-Abl tyrosine kinase, and could significantly improve patients’ survival [13,14]. This study utilized optimal treatment plans for patients depending on their disease phase. However, drug resistance was observed in certain CML patients, thus affecting treatment efficacy, making it a major challenge in CML treatment.
Immune system plays an important role in the clearance of tumor cells by body defence mechanism. During the malignant transformation of normal cells into tumors, certain immune response can be activated by body immune system to eliminate such potentially tumor cells. When few tumor cells “escape” the immune surveillance immune by certain mechanisms, however, they developed into cancer. The mechanism of such immune escape is still unclear yet. Recent study suggested that CD4+CD25+ Treg might be one of the important mechanisms participating in immune escape of tumor cells [15]. CD4+CD25+ Treg is a T cell sub-population with potentially immune modulation function. It can actively inhibit T cell immune function, and plays important roles in maintaining autoimmune function, preventing autoimmune disease and tumor immune response. Various studies showed the close correlation between Treg and tumor immunity. In various tumors such as pulmonary carcinoma, breast cancer, gastrointestinal tumor and hematological tumor, the expression of CD4+CD25+ Treg was significantly elevated, indicating its correlation with tumor pathogenesis [16-18]. Shenghui et al. found remarkably higher CD4+CD25+ Treg ratio in all CD4+ T cells in acute leukemia patients compared with control people, plus its correlation with unfavorable prognosis [19]. Forkhead transcription factor 3 (FOXP3) is a member of forkhead transcriptional factor family. Study has showed its specific expression in CD4+CD25+ Treg, and its important role in cell development and functional maintenance, making it as a specific marker for CD4+CD25+ Treg [20]. Therefore, this study utilized CD4+, CD25+ and Foxp3+ as three parameters for evaluating Treg, which has higher accuracy and specificity compared with previous literatures [21]. Treg exerts immune suppressant effects via direct contact with cells or via autocrine cytokines, thus disrupting critical immune cell functions in tumor focal region or micro-vessels, thus inhibiting anti-tumor immune response of tumor patients. Wu et al. found significantly elevated CD4+CD25+ Treg level in peripheral blood from acute lymphatic leukemia patients, which also had elevated serum IL-10 and TGF-beta levels but lowered IL-6 level, suggesting the effect of CD4+CD25+ Treg on immune response via affecting the secretion of immune cytokines including IL-10 and IL-2 [21]. Our study on CML patients also revealed higher CD4+CD25+ Treg level in T cells compared with control group.
Currently there were few reports on the effect of CD4+CD25+ Treg on the prognosis and survival of CML patients. Rojas et al. found significantly lower CD4+CD25+Foxp3+ Treg in all CD4+T cells in CCR patients compared to those without remission [22]. The author believed that Treg might inhibit body immune response against Bcr-Akl. Our study found significantly higher CD4+CD25+ Treg level in peripheral blood samples of CML patients, plus variations across different disease stages. During chronic untreated and accelerated blast phase, CD4+CD25+ Foxp3+ Treg ratio was higher than that in chronic treated group. However, no significant difference existed between chronic untreated and accelerative/blast group. These results suggested that when CML disease condition was stable, CD4+CD25+Foxp3+ Treg level was significantly lowered compared to those before treatment, consistent with previous studies. Using averaged ratio of CD4+CD25+Foxp3+ Treg in all CD4+ cells as the cut-off line, CML patients were further divided into high level and low level group. CD4+CD25+Foxp3+ Treg in high level group revealed lower 3mCHR, 12mCCR and 18mCMR compared to low-level group, plus higher incidence of drug resistance. Further survival analysis revealed significantly lower survival rate in those with CD4+CD25+FoxP3 Treg high expression. Some scholars believed that Treg mainly participates in tumor escape process via secreting inhibitory cytokines such as IL-10 and TGF-beta [23]. Chen et al. found that CD4+CD25+Treg may inhibit CD8+ T cell induced tumor clearance function [24]. Miao et al. revealed that Treg could activate Th1 pathway and inhibited Th2 induced DC-CIK cell’s anti-tumor effects [15]. Clinical studies also found high expression levels of Treg in lymph node, tumor region and peripheral blood of various cancer patients. Such elevated Treg cells could affect tumor progression, suppressing tumor treatment efficacy, and elevated recurrent risk [25,26]. The elimination or blocking Treg to inhibit their functions can recover tumor immunity to certain extents [27,28]. Therefore the decrease of high level status of CD4+CD25+FoxP3 Treg via certain ways might be a promising approach to treat malignant tumors.
Conclusion
This study investigated the expression of CD4+CD25+FoxP3 Treg in different phases of CML, and found adverse effects of high level of CD4+CD25+FoxP3 Treg on patient survival rate and prognosis, thus providing evidences and targets for CML treatment in clinic.
References
- DeAngelo DJ, Stein EM, Ravandi F. Evolving therapies in acute myeloid leukemia: progress at last? Am Soc Clin Oncol Educ Book 2016; 35: 302-312.
- Jindra P. Prognostic factors to predict outcome of reduced intensity allogeneic haematopoietic cell transplantation for chronic lymphocytic leukemia. Neoplasma 2016; 63: 595-600.
- Pettit K, Stock W, Walter RB. Incorporating measurable ('minimal') residual disease-directed treatment strategies to optimize outcomes in adults with acute myeloid leukemia. Leuk Lymphoma 2016; 57: 1527-1533.
- Paul S, Lal G. Regulatory and effector functions of gamma-delta (gammadelta) T cells and their therapeutic potential in adoptive cellular therapy for cancer. Int J Cancer 2016; 139: 976-985.
- Beyer M. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood 2006; 107: 3940-9.
- Wang X. Increased population of CD4 (+) CD25 (high), regulatory T cells with their higher apoptotic and proliferating status in peripheral blood of acute myeloid leukemia patients. Eur J Haematol 2005; 75: 468-476.
- Perrone S. How has treatment changed for blast phase chronic myeloid leukemia patients in the tyrosine kinase inhibitor era? A review of efficacy and safety. Expert Opin Pharmacother 2016; 17: 1517-1526.
- Miranda MB. Secondary malignancies in chronic myeloid leukemia patients after imatinib-based treatment: long-term observation in CML Study IV. Leukemia 2016; 30: 1255-1262.
- Perrotti D. Cellular and molecular networks in chronic myeloid leukemia: the leukemic stem, progenitor and stromal cell interplay. Curr Drug Targets 2017; 18: 377-388.
- Burchert A, Neubauer A. Chronic myeloid leukemia: Diagnostics, therapy and future strategy. Internist (Berl) 2011; 52: 283-293.
- Maru Y. Molecular biology of chronic myeloid leukemia. Cancer Sci 2012; 103: 1601-1610.
- Comert M, Baran Y, Saydam G. Changes in molecular biology of chronic myeloid leukemia in tyrosine kinase inhibitor era. Am J Blood Res 2013; 3: 191-200.
- Ali MA. Chronic myeloid leukemia in the era of tyrosine kinase inhibitors: an evolving paradigm of molecularly targeted therapy. Mol Diagn Ther 2016; 20: 315-333.
- Al-Achkar W. Correlation of p210 BCR-ABL transcript variants with clinical, parameters and disease outcome in 45 chronic myeloid leukemia patients. J Buon 2016; 21: 444-449.
- Miao L, Run-Ming J, Yi J. T-Bet mediated anti-neoplastic effects of dendritic cell-cytokine induced killer cells in vitro. Iran J Pediatr 2012; 22: 43-51.
- Wolf AM. Increase of regulatory T cells in the peripheral blood of cancer patients. Clin Cancer Res 2003; 9: 606-612.
- Geis AL, Housseau F. Procarcinogenic regulatory T cells in microbial-induced colon cancer. Oncoimmunol 2016; 5: e1118601.
- Lauricella M. The analysis of estrogen receptor-alpha positive breast cancer stem-like cells unveils a high expression of the serpin proteinase inhibitor PI-9: Possible regulatory mechanisms. Int J Oncol 2016; 49: 352-360.
- Shenghui Z. Elevated frequencies of CD4 (+) CD25 (+) CD127lo regulatory T cells are associated to poor prognosis in patients with acute myeloid leukemia. Int J Cancer 2011; 129: 1373-1381.
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4: 330-336.
- Wu CP. Immunophenotype and increased presence of CD4 (+) CD25 (+) regulatory T cells in patients with acute lymphoblastic leukemia. Oncol Lett 2012; 3: 421-424.
- Rojas JM. Naturally occurring CD4+ CD25+ FOXP3+ T-regulatory cells are increased in chronic myeloid leukemia patient’s not in complete cytogenetic remission and can be immunosuppressive. Exp Hematol 2010; 38: 1209-1218.
- Michael M, Shimoni A, Nagler A. Regulatory T cells in allogeneic stem cell transplantation. Clin Dev Immunol 2013; 2013: 608951.
- Chen ML. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA 2005; 102: 419-424.
- Schuler PJ. Dendritic cell generation and CD4+ CD25 high FOXP3+ regulatory t cells in human head and neck carcinoma during radio-chemotherapy. Eur J Med Res 2011; 16: 57-62.
- Kindlund B. CD4+ regulatory T cells in gastric cancer mucosa are proliferating and express high levels of IL-10 but little TGF-beta. Gastric Cancer 2017; 20: 116-125.
- Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol 2016; 28: 401-409.
- Chellappa S. Regulatory T cells that co-express ROR gammat and FOXP3 are pro-inflammatory and immunosuppressive and expand in human pancreatic cancer. Oncoimmunol 2016; 5: e1102828.