- Biomedical Research (2014) Volume 25, Issue 1
The enhanced apoptotic effect of photodynamic therapy using photofrin combined with genistein in human ovarian cancer cell SK-OV-3.
Jin-Chul Ahn1,2, Raktim Biswas1, Jong-Soo Kim3,*1Beckman Laser Institute Korea, Dankook University, Cheonan, Chungnam 330-715, Korea.
2Department of Biomedical Engineering, Dankook University, Cheonan, Chungnam 330-715, Korea.
3Department of Obstetrics & Gynecology, College of Medicine, Dankook University, Cheonan, Chungnam 330-715, Korea.
- *Corresponding Author:
- Jong-Soo Kim
Department of Obstetrics & Gynecology
College Of Medicine, Dankook University
359 Manhyangro, Dongnan-gu, Cheonan
Chungcheongnam-do, 330-715
Republic of Korea.
Accepted Date: September 12, 2013
Abstract
Photodynamic therapy (PDT) has become one of the popular treatment modalities for the management of cancer and other diseases. PDT in combination with conventional chemotherapy has shown an enhanced efficacy in combating different types of cancers. In this present study, we investigated the cytotoxic and apoptotic effects of genistein in combination with photofrin induced PDT in an ovarian cancer cell line SK-OV-3. Cells were treated with different concentrations of genistein (0, 25, 50, and 100μM) in combination with 3.13μg/ml and 6.25 μg/ml of phtofrin induced PDT. The viabilities of the cells were decreased by 34.5% and 49.0% respectively with the different concentrations of photofrin in PDT. In order to investigate whether genistein in combination with PDT can induce apoptotis, SK-OV-3 cells were stained with hoechst 33342 and propidium iodide. From the microscopic images, it was observed that apoptosis was induced in the SK-OV-3 cells treated with either genistein (100μM) or PDT alone. In addition, different apoptosis related proteins like caspase-8, caspase-9, caspase-3, cytochrome c and PARP were also activated when the cells were treated with the combination of genistein and PDT. These results show that the general apoptotic pathway requiring caspase-3 activation through caspase-8 is the main pathway that induces apoptosis in SK-OV-3 cells. Therefore, we conclude that genistein sensitizes the activity of photodynamic therapy by photofrin in SK-OV-3 cells by inducing apoptosis through the activation of caspase-8 and caspase-3.
Keywords
Apoptosis, Genistein, SK-OV-3 (ovarian cancer cells), PDT, Photofrin.
Introduction
Ovarian cancers are usually diagnosed at advanced stages with massive and widely spread intra abdominal diseases. The prognosis for advanced stage ovarian cancer is very poor. The 5-year survival rate for stage III A is 41%, for stage IIIB is about 25%, for stage IIIC is 23%, and for stage IV disease is 11% [1].
Photodynamic therapy (PDT) is becoming widely accepted as a potential treatment for cancer and other nonmalignant diseases. In PDT, the photosensitizer can be concentrated into the tumor tissue and can be excited by a light of specific wavelength. Therefore, the subsequent photodynamic reaction can generate cytotoxic reactive oxygen species (ROS) and lead to the destruction of cancer cells [2,3]. In the last few years, photofrin has been approved for treating various forms of premalignant and malignant diseases such as the cancer of the treatment of skin, cervix, lung and esophagus [3-5].
Genistein is a natural component of soybean and is known as 4’,5,7 trihydroxy isoflavons which is a class of flavinoids. Genistein has been found to be a potent anticancer agent [6]. This isoflavone is a phytoestrogen that occurs naturally in the diet and is found in a wide variety of foods derived from plants especially in soybeans and soybased foods [7]. Some of the potential biological effects of phytoestrogens, especially, genistein, include inhibition of angiogenesis in malignant tumours, inhibition of the activity of tyrosine kinase and human DNA topoisomerase II activity [6,8,9]. Genistein induces G2/M cell cycle arrest and apoptosis of human overian cancer cells. It also inhibits the autophosphorylation of the epidermal growth factor receptor, a receptor which is overexpressed in most cancers [10,11]. Beside this genistein acts as an antioxidant, thus preventing oxidative DNA damage; and cause changes characteristic of apoptosis, a protective mechanism induced in damaged, mutated, or cancerous cells [12,13]. It was also found to be effective against human anaplastic thyroid cancer cells [14]. Gynecologically, genistein is commonly used as a non-invasive chemopreventive agent that can modulate several proteins like Bax, Bcl-2, cytochrome c and different caspases like caspase 3, caspase 8 to induce apoptosis and thereby can control growth in cervical cancer cells [15].
Nowadays, combination of two different modalities is being applied for the treatment of various types of cancer. This combination modality can not only enhance the efficacy of the treatment but also can reduce the doses of chemotherapy drugs and thus can reduce their side effects. Several studies have confirmed the higher efficacy of natural components against different types of cancer when they were treated in combination with other modalities like photodynamic therapy [14,16].
In this present study, we investigated the molecular mechanism by which genistein enhances the anticarcinogenic effect in cultured SK-OV-3 ovarian cells when treated in combination with photodynamic therapy.
Materials and Methods
Chemicals and antibodies
The photosensitizer, Photofrin was provided by Kumho Life and Environmental Science Laboratory (Kwangju, Korea). A stock solution (1.5 mg/ml) was prepared in ethanol and kept at -20ºC in dark. Photofrin was diluted in the culture medium just before use and the cells were treated at their exponential growth phase.
Roswell Park Memorial Institute medium (RPMI), fetal bovine serum (FBS), and Dulbecco's Phosphate-Buffered Saline (DPBS) were purchased from Hyclone (South Logan, UT, USA). The antibodies were purchased as follows: - anti cytochrome c from BD Biosciences Pharmingen, San Diego, CA, USA, anti-caspase-3 and -8 from Calbiochem, Darmstadt, Germany, anti-PARP (poly ADP ribose polymerase) from Cell Signaling, Danvers, MA, USA. MTT (3-4,5-dimethylthiazol-2-yl-2,5- diphenyltetrazoilium bromide), Hoechst 33342 and genistein were purchased from Sigma, St. Louis, MO, USA. Stock solutions of 1mM genistein were prepared in dimethylsulfoxide (DMSO) and stored in the dark at -20ºC.
Cell culture
SK-OV-3 is a human ovarian cancer cell line with epithelial like morphology. SKOV-3 cells can form moderately well differentiated adenocarcinoma consistent with ovarian primary cells in vivo. This cell line is produced by the Korean Cell Line Bank (Seoul, South Korea). Cells (2×105 cells/well) were cultured in RPMI medium containing 10% FBS and 1% antibiotics (streptomycin and penicillin) at 37ºC in a 5% CO2 humidified atmosphere and then seeded into a 96-well microplates for 24 hours. For the PDT treatment, cells were treated with increasing concentrations (0-50μg/ml) of photofrin. Then, the photosensitized cells were exposed to 632 nm diode laser at an intensity of 2.0 J/cm2 for 15 min to activate the photofrin. After irradiation, the cells were incubated at 37ºC in the dark for a period of 3 to 24 hrs and the several parameter were studied at various time points required. Again to study the combination effect, cells were treated with different concentrations of genistein (0, 50, 100 μM) and increasing concentrations of photofrin (0-50μg/ml) for PDT.
MTT assay
The viabilities of the control and treated cells were measured by the MTT assay method. Briefly, cells were seeded in a 96-well culture plate at a concentration of 1×105 cells/well and treated with photofrin PDT and combination of PDT with genistein as mentioned before. After photosensitization, the cells were incubated with MTT solution (2 mg/ml) for 2 hours at 37ºC. The culture medium was then discarded and 200μl of Dimethyl sulfoxide (DMSO) was added to each well. The optical densities in the 96-well plates were determined with a microplate reader (Bio-Rad, Hercules, CA, USA) at 540 nm. The percentage of cell viability was calculated as below;
The percentage of cell viability (%) = mean absorbance (treatment)/Mean absorbance (control) ×100.
Detection of apoptosis using Hoechst 33342 and PI double staining
Apoptosis was observed by chromatin staining with Hoechst 33342. Cells were seeded on a cover slips in a 6- well plate. After laser irradiation for PDT, the supernatant was discarded and the cells were fixed with 4% paraformaldehyde in PBS for 1 hour at room temperature. Then the cells were washed three times in PBS and stained with Hoechst 33342 (Sigma, St. Louis, MO, USA) at 10 μM for 1 hour at room temperature. The culture medium was changed to a fresh one and the cells were incubated with Propidium Iodide (PI) (2 μM) for 10 min. Fluor-stained nuclei were observed using a Zeiss confocal microscope (Zeiss, Oberkochen, Germany) with the FITC filter set to detect the apoptotic cells with condensed nuclei.
Western blot
After treating the SK-OV-3 cells with PDT, genistein and combination of photofrin-PDT and genistein; the expressions of caspase-8, caspase-9, cytochrome c, caspase-3, PARP (poly ADP-Ribose ploymerase), were detected by Western blot analysis. GAPDH and β-actin were used as loading controls. After treatments, cells were rinsed twice with ice-cold PBS. Then the cells were collected by scraping the bottom of the culture plate and harvested by centrifugation at 1000 rpm for 5 min. The cells were then lysed with ice cold RIPA buffer (Sigma, St. Louis, MO, USA) containing phosphatase and protease inhibitors. Solubilized proteins were again centrifuged at 14,000 rpm for 30 min, and the supernatant was collected. The protein concentration was determined using the Bradford assay reagent (Bio-Rad, Hercules, CA). Equal amounts of protein were separated by 10% sodium dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE) and transferred to immunoblot PVDF membranes (Bio-Rad, Hercules, CA). Electrophoresis and blotting were both performed using the Power Pac200 electrophoresis system (Bio-Rad, Hercules, CA). After blocking with 5% non-skimmed milk for 1 hour, the membrane was incubated with the primary antibodies overnight at 4ºC. The membranes were blocked again with 5% skim milk in PBST, and incubated with antibodies against caspase-8, casepase-9, cytochrome c, caspase-3, PARP and β-actin. Immunocomplexes were detected by subsequent incubation with appropriate horseradish peroxidase-conjugated secondary IgG antibodies, and with enhanced chemiluminescence (ECL), according to the manufacturer protocol (Amersham Pharmacia Biotechnology, Buckinghamshire, UK). The protein bands were detected by the Kodak image analyzer.
Results
MTT assay
Genistein-sensitized SK-OV-3 cells to PDT induced growth inhibition
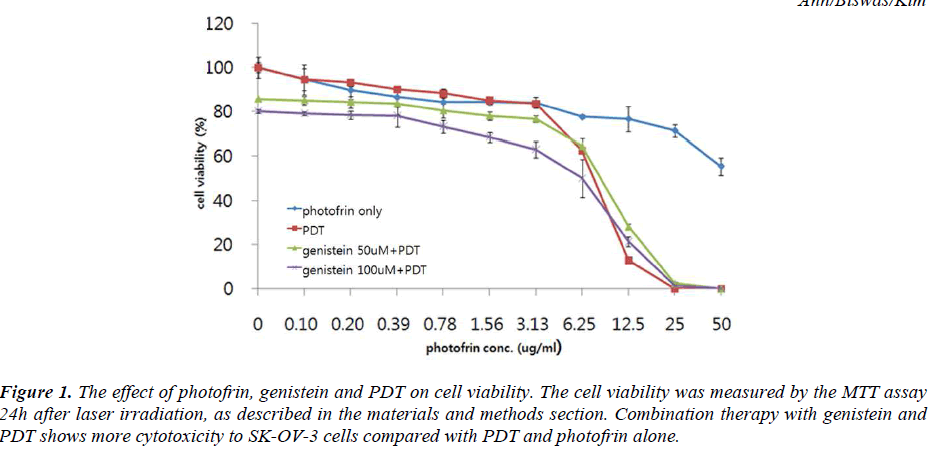
The effect of different concentrations of photofrin PDT on the viability of SK-OV-3 cells was determined by the MTT assay. Results showed a concentration-dependent inhibition of cell viability by PDT treatment. Subsequent studies were undertaken to determine whether cells preconditioned with genistein were more sensitive to the cytotoxic effect of PDT using the cell viability assay. The effect of genistein and PDT on the viability of the SKOV- 3 cells is illustrated in Fig. 1. When the cells were treated with 100μM genistein followed by 3.13μg/ml and 6.25μg/ml of photofrin induced PDT, the percentages of viability of the cells were decreased by 34.5% and 49.0% respectively. These results suggest that a combination of genistein with photofrin elicits a significantly greater inhibition of the viability of the SK-OV-3 cells as compared to individual treatment alone.
Figure 1: The effect of photofrin, genistein and PDT on cell viability. The cell viability was measured by the MTT assay 24h after laser irradiation, as described in the materials and methods section. Combination therapy with genistein and PDT shows more cytotoxicity to SK-OV-3 cells compared with PDT and photofrin alone.
Genistein-sensitized SK-OV-3 cells undergo apoptosis and necrotic cell death induced by PDT.
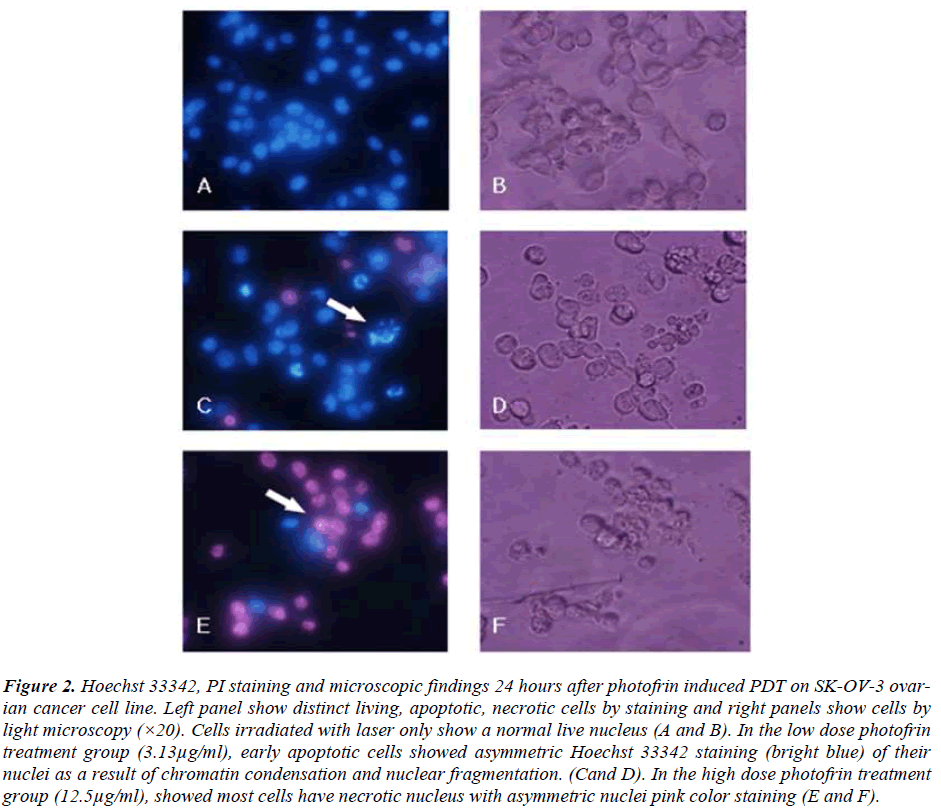
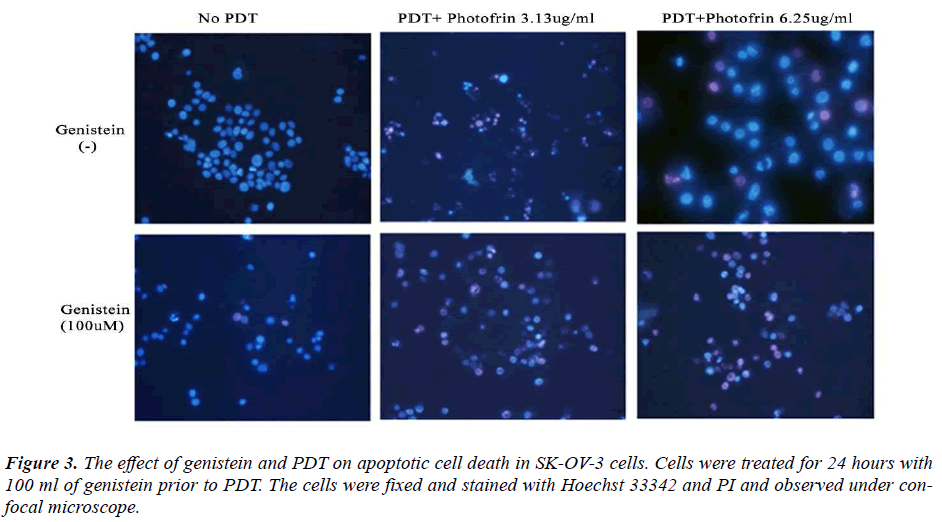
In order to investigate whether genistein and PDT induced apoptosis, SK-OV-3 cells were stained with Hoechst 33342. Hoechst 33342 is a fluorescent dye that specifically stains nucleic acid. Normal cells stained with Hoechst 33342 showed normal morphology of nuclei (blue), while apoptotic cells showed shrinkage or condensation of the nuclei (bright blue). From figure 2, normal live nucleus can be observed in the cells treated with lasers only (A and B). In the low dose of photofrin (3.13μg/ml) treatment in PDT group, early apoptotic cells can be observed (bright blue) with cell blebbing, chromatin condensation and nuclear fragmentation (C and D). In the high dose photofrin treatment group (12.5μg/ml), most cells have necrotic nucleus with asymmetric nuclei with pink color staining (E and F). Therefore, for the cells treated with higher concentrations of photofrin PDT, late apoptotic and necrotic cells can be observed by propidium iodide (PI) (Fig. 2). From the microscopic images of the cells, we observed induction of apoptosis in the SK-OV-3 cells treated with either genistein (100μM) and PDT (3.13μg/ml and 6.25μg/ml) or combination of genistein and PDT (Fig. 3). Pretreatment with genistein followed by PDT, induced higher rate of apoptosis in the SK-OV-3 cells compared to the individual treatments. These results are consistent with the data obtained for inhibition of cell viability by MTT, suggesting that the loss of viability of cells treated with genistein followed by PDT compared much higher to the individual treatments. Therefore, this combination treatment has higher efficacy of inducing apoptosis in SK-OV-3 cell.
Figure 2: Hoechst 33342, PI staining and microscopic findings 24 hours after photofrin induced PDT on SK-OV-3 ovarian cancer cell line. Left panel show distinct living, apoptotic, necrotic cells by staining and right panels show cells by light microscopy (×20). Cells irradiated with laser only show a normal live nucleus (A and B). In the low dose photofrin treatment group (3.13μg/ml), early apoptotic cells showed asymmetric Hoechst 33342 staining (bright blue) of their nuclei as a result of chromatin condensation and nuclear fragmentation. (Cand D). In the high dose photofrin treatment group (12.5μg/ml), showed most cells have necrotic nucleus with asymmetric nuclei pink color staining (E and F).
Genistein augmented the apoptosis signaling pathways which were stimulated when SK-OV-3 cells were treated with PDT.
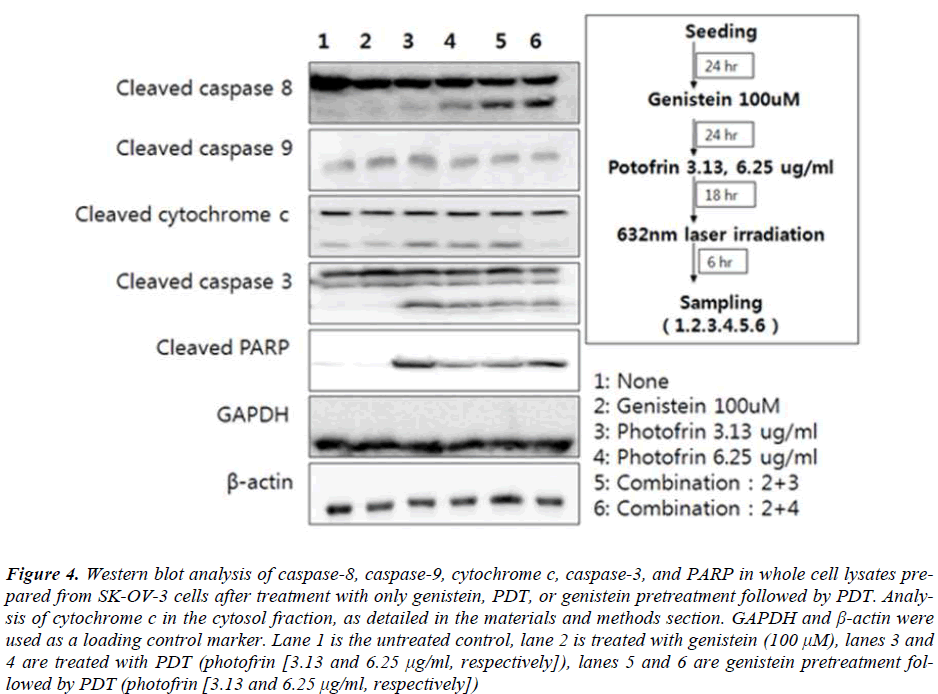
Caspases are cysteine proteases which are activated during the apoptotic signaling pathways Specific caspases are used as initiators and executors by different apoptotic signaling pathways. Treatment of SK-OV-3 cells with genistein or a low concentration of photofrin induced PDT failed to show cleavage of caspase-8, while the highconcentration of photofrin induced PDT alone showed expression of cleaved caspase-8 (Fig. 4). On the other hand combination with genistein substantially augmented the level of expression of cleaved caspase-8 (Fig. 4). To analyze the involvement of mitochondrial pathway related protein in induction of apoptosis in SK-OV-3 cells by genistein and PDT, expressions of different apoptotic protens like cytochrome c, Caspase 9, Caspase 3 and PARP were analysed. Protein from cytosolic fractions were prepared and analyzed for the levels of cytochrome c. From the results, it was observed that the expression of cytochrome c was upregulated with the increasing concentrations of photofrin in PDT in combination group. The expression of caspase-9 was also upregulated in PDT treated groups, however the combination of PDT with genistein did not affect the level of the expression of caspase-9. On the other hand, the expression of caspase-3 and PARP were much higher in the cells treated with genistein and high concentration of photofrin induced PDT.
Figure 4: Western blot analysis of caspase-8, caspase-9, cytochrome c, caspase-3, and PARP in whole cell lysates prepared from SK-OV-3 cells after treatment with only genistein, PDT, or genistein pretreatment followed by PDT. Analysis of cytochrome c in the cytosol fraction, as detailed in the materials and methods section. GAPDH and β-actin were used as a loading control marker. Lane 1 is the untreated control, lane 2 is treated with genistein (100 μM), lanes 3 and 4 are treated with PDT (photofrin [3.13 and 6.25 μg/ml, respectively]), lanes 5 and 6 are genistein pretreatment followed by PDT (photofrin [3.13 and 6.25 μg/ml, respectively])
Discussion
PDT is a clinically-approved cancer treatment method, which is expected to eliminate the microscopic tumors. Genistein, the phytoestrogen present in soybeans, has a wide range of properties that may contribute to cancer protective actions, such as an inhibitory effect on tyrosine kinases, antiestrogenicity, antioxidant activity, antiangiogenesis activity, suppression of cell proliferation, induction of differentiation, and modulation of apoptosis [11-14,17]. This natural component of soy has recently been found to be of chemotherapeutic value when combined with tumor-specific antibodies in the treatment of B-cell precursor leukaemia [13].
Recent research has shown that genistein is a chemopreventive agent which mainly reactivate estrogen receptor to enhance hormonal therapy of breast cancer [18]. It also upregulate tumor suppressor microRNA in prostate cancers [19]. Epidemiological studies also provide evidence for the prevention of breast cancer through consumption of soybeans and specifically soy isoflavones [20,21]. Two distinctive types of biological responses have been reported in genistein treated tumor cells. The first type is antiproliferative, cytostatic, and cell differentiating effects, which have been reported at relatively low genistein concentrations ranging from 10 to 45mM [22-24]. The second type of biological response is the cytotoxic effect [25]. Based on its ability to induce apoptosis in HL- 60 cells, B-cell precursor leukemia cells and Jurkat Tleukemia cells, genistein has been evaluated as an apoptotic agent for different types of cancer [26-28]. Although the mechanism of its action has been studied extensively, the sequence of molecular events involved in the apoptotic response is still subject to speculation.
Therefore, the aim of our study was to determine the combined effect of PDT and genistein, in order to reduce the effective dosage of the individual compounds and therefore to reduce their side effects on normal cells. The enhanced apoptotic effects and the possible mechanism of action of this enhanced efficacy has been studied. The combined treatment of PDT and genistein significantly inhibited cell proliferation and enhanced apoptosis in SKOV- 3 cells compared to the individual treatment with PDT or genistein.
Most photosensitizers for PDT bind to mitochondria, lysosomes, and/or other intracellular membranes, including the ER [29]. Photoactivation of a mitochondrion-localized photosensitizer causes the depolarization of mitochondrial membrane potential nad release of cytochrome c. The released cytochrome c helps in generating active caspase- 9, which then cleaves and activates caspase-3. Caspase-3 is the major effector caspase and is responsible for cleavage and activation of other caspases, especially caspases- 6, -7, and -8 [30]. Our results showed activation of caspase- 8, caspase-9, caspase-3, and PARP after combined treatment with PDT and genistein. These results indicate that the apoptotic pathway requiring caspase-3 activation through caspase-8 is the main pathway to induce apoptosis in SK-OV-3 cells.
Therefore, we can conclude that combined treatment with PDT and genistein results in an enhanced cytotoxic and apoptotic effect in SK-OV-3 cells through activation of caspase-8, caspase-3. Though further in vivo and preclinical studies are required, this study may shed a light to enhance the efficacy of chemotherapy drugs by combination treatment with photodynamic therapy.
Acknowledgement
The present research was conducted by the research fund of Dankook University in 2012.
References
- Kosary CL Cancer of the ovary. Survellance Survival and End Results (SEER) survival monograph. J Natl Cancer Inst. 2007; 16:133-144.
- Castano AP, Demidova TN and Hamblin MR. Mechanisms in photodynamic therapy: part two— cellular signaling, cell metabolism and modes of cell death. Photodiagnosis and photodynamic therapy 2005; 2:1-23.
- Ortel B and Calzavara-Pinton P. Advances in photodynamic therapy. A review. Giornale italiano di dermatologia evenereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia 2010; 145:461- 475.
- Dolmans DE, Fukumura D and Jain RK. Photodynamic therapy for cancer. Nature reviews. Cancer 2003 3:380-387.
- Corti L, Mazzarotto R, Belfontali S, De Luca C, Baiocchi C, Boso C and Calzavara F. Photodynamic therapy in gynaecological neoplastic diseases. Journal of photochemistry and photobiology. B, Biology 1996; 36:193-197.
- Fotsis T, Pepper M, Adlercreutz H, Hase T, Montesano R and Schweigerer L. Genistein, a dietary ingested isoflavonoid, inhibits cell proliferation and in vitro angiogenesis. The Journal of nutrition 1995; 125:790S- 797S.
- Fukutake M, Takahashi M, Ishida K, Kawamura H, Sugimura T and Wakabayashi K. Quantification of genistein and genistin in soybeans and soybean products. Food and Chemical Toxicology 1996; 34:457-461.
- Yan GR, Xiao CL, He GW, Yin XF, Chen NP, Cao Y and He QY. Global phosphoproteomic effects of natural tyrosine kinase inhibitor, genistein, on signaling pathways. Proteomics 2010; 10:976-986.
- Mizushina Y, Shiomi K, Kuriyama I, Takahashi Y and Yoshida H. Inhibitory effects of a major soy isoflavone, genistein, on human DNA topoisomerase II activity and cancer cell proliferation. International journal of oncology 2013; 43:1117-1124.
- Qi W, Weber C, Wasland K and Savkovic S. Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC cancer 2011; 11:219..
- Ouyang G, Yao L, Ruan K, Song G, Mao Y and Bao S. Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell biology international 2009; 33:1237-1244.
- Darbon JM, Penary M, Escalas N, Casagrande F, Goubin-Gramatica F, Baudouin C and Ducommun B. Distinct Chk2 activation pathways are triggered by genistein and DNA-damaging agents in human melanoma cells. The Journal of biological chemistry 2000; 275:15363-15369.
- Bingham SA, Atkinson C, Liggins J, Bluck L and Coward A. Phyto-oestrogens: where are we now? The British journal of nutrition 1998; 79:393-406.
- Ahn JC, Biswas R and Chung PS. Combination with genistein enhances the efficacy of photodynamic therapy against human anaplastic thyroid cancer cells. Lasers in surgery and medicine 2012; 44:840-849.
- Kim SH, Lee SC and Song YS. Involvement of both extrinsic and intrinsic apoptotic pathways in apoptosis induced by genistein in human cervical cancer cells. Annals of the New York Academy of Sciences 2009; 1171:196-201.
- Ahn J-C, Biswas R and Chung P-S. Synergistic effect of radachlorin mediated photodynamic therapy on propolis induced apoptosis in AMC-HN-4 cell lines via caspase dependent pathway. Photodiagnosis and photodynamic therapy 2013; 10:236-243.
- Chang KL, Kung ML, Chow NH and Su SJ. Genistein arrests hepatoma cells at G2/M phase: involvement of ATM activation and upregulation of p21waf1/cip1 and Wee1. Biochemical pharmacology 2004; 67:717-726.
- Li Y, Meeran SM, Patel SN, Chen H, Hardy TM and Tollefsbol TO. Epigenetic reactivation of estrogen receptor-alpha (ERalpha) by genistein enhances hormonal therapy sensitivity in ERalpha-negative breast cancer. Molecular cancer 2013; 12:9..
- Chiyomaru T, Yamamura S, Fukuhara S, Hidaka H, Majid S, Saini S, Arora S, Deng G, Shahryari V, Chang I, Tanaka Y, Tabatabai ZL, Enokida H, Seki N, Nakagawa M and Dahiya R. Genistein Up-Regulates Tumor Suppressor MicroRNA-574-3p in Prostate Cancer. PLoS ONE 2013; 8:e58929..
- Lamartiniere CA Protection against breast cancer with genistein: a component of soy. The American Journal of Clinical Nutrition 2000; 71:1705s-1707s.
- Peterson G and Barnes S. Genistein inhibition of the growth of human breast cancer cells: Independence from estrogen receptors and the multi-drug resistance gene. Biochemical and Biophysical Research Communications 1991; 179:661-667.
- Choi E, & Kim, G. Antiproliferative activity of daidzein and genistein may be related to ERα/c-erbB-2 expression in human breast cancer cells. Molecular Medicine Reports 2013; 7(3):781-784.
- Kayisli UA, Guzeloglu-Kayisli O, Guzel E and Arici A. Genistein Inhibits Cell Proliferation and Stimulates Apoptosis in Human Coronary Artery Endothelial Cells. Gynecologic and Obstetric Investigation 2013; 75:235- 242.
- Dong X, Xu W, Sikes RA and Wu C. Combination of low dose of genistein and daidzein has synergistic preventive effects on isogenic human prostate cancer cells when compared with individual soy isoflavone. Food Chemistry 2013; 141:1923-1933.
- Hussain A, Harish G, Prabhu SA, Mohsin J, Khan MA, Rizvi TA and Sharma C. Inhibitory effect of genistein on the invasive potential of human cervical cancer cells via modulation of matrix metalloproteinase-9 and tissue inhibitiors of matrix metalloproteinase-1 expression. Cancer Epidemiology 2012; 36:e387-e393.
- 26. Li Q-S, Li C-Y, Li Z-L and Zhu H-L. Genistein and its Synthetic Analogs as Anticancer Agents. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry 2012; 12:271-281.
- Uckun F, Evans W, Forsyth C, Waddick K, Ahlgren L, Chelstrom L, Burkhardt A, Bolen J and Myers D. Biotherapy of B-cell precursor leukemia by targeting genistein to CD19-associated tyrosine kinases. Science (New York, N.Y.) 1995; 267:886-891.
- Spinozzi F, Pagliacci MC, Migliorati G, Moraca R, Grignani F, Riccardi C and Nicoletti I. The natural tyrosine kinase inhibitor genistein produces cell cycle arrest and apoptosis in Jurkat T-leukemia cells. Leukemia Research 1994; 18:431-439.
- Ahn JC, Biswas R, Moon JH and Chung PS. Cellular uptake of 9-hydroxypheophorbide-α and its photoactivation to induce ER stress-related apoptosis in human cervical cancer cells. Lasers Med Sci 2013;
- Costantini P, Jacotot E, Decaudin D and Kroemer G.Mitochondrion as a novel target of anticancer chemotherapy. Journal of the National Cancer Institute 2000; 92:1042-1053.