- Biomedical Research (2011) Volume 22, Issue 1
The efficacy of Royal Jelly in the restoration of alcoholic liver injury in mouse model
Changchun Li1,3*, Md. Kaiissar Mannoor2, Nami Toma3, Tomoyo Taniguchi4, Masashi Inafuku3, Ki-kuji Yamaguchi5, 6, Yoshiya Sato2 and Hisami Watanabe3
1Transdisciplinary Research Organization for Subtropics and Island Studies, University of the Ryukyus, Okinawa 903-0213, Japan
2Department of Parasitology and International Health, Graduate School of Medicine, University of the Ryukyus, Okinawa 903-0215, Japan
3Immunobiology Group, Center of Molecular Biosciences, Tropical Biosphere Research Center, University of the Ryu-kyus, Okinawa 903-0213, Japan
4Division of Medical and Technological Sciences Graduate School of Health Sciences, Niigata University, Niigata 951-8518, Japan
5Japan Royal Jelly (JRJ) Co. Ltd., Tokyo 163-0517, Japan
6Apis Cerana Research Institute, Yunnan Agricultural University, Yunnan 650201, China
- *Corresponding Author:
- Changchun Li
Transdisciplinary Research Organization
for Subtropics and Island Studies
University of the Ryukyus
Okinawa 903-0213
Japan
Accepted date:September 21 2010
Abstract
Prolonged excessive ingestion or consumption of alcohol eventually results in hepatic insuffi-ciency; induces the aggravation of viral hepatitis and/or fatty liver. The long term ingestion of alcohol not only induces a decline in the immune function but also promotes the produc-tion of inflammatory cytokines by Kupffer cells activated by enterobacterial endotoxins. Royal Jelly has numerous functions, such as for the maintenance of health, immunopotentia-tion and age-retarding etc. Furthermore, Royal Jelly functions as a potent immunomodula-tor, such as Royal Jelly ameliorates stress-associated immune dysfunction. An alcoholic he-patic insufficiency mouse model was constructed by feeding it a liquid diet containing 5 % ethanol and features of the innate immune system were observed to assess whether Royal Jelly has any effect on alcohol-induced liver insufficiency. The data reveal that Royal Jelly administration (1) exhibits prophylactic effect on alcohol-induced hepatomegaly and (2) functions in restoration of transaminase levels caused by impaired hepatocytes. Royal Jelly-modulated important immune phenomena on alcoholic liver injury include (1) activation of liver natural killer cells and (2) control of the levels of IL-4, IL-5, and TNF-alpha in the se-rum. These findings provide evidence that Royal Jelly might have the capacity to restore the function of the immune system in individuals with alcoholic liver diseases.
Key words
Royal Jelly, alcoholic liver injury, immunoregulation, cytokine.
Introduction
Royal Jelly (RJ) is a creamy substance secreted from hy-popharyngeal glands and mandibular glands of worker bees. Nectar is the crude material of the RJ, and RJ is a special food fed to the larvae and adult queens. RJ is rich in vitamins, minerals, amino acids, short chain fatty acids and a variety of other nutrients including 12 % - 15 % crude proteins.
RJ is a time-honored Chinese remedy known to have nu-merous functions, such as maintenance of health, im-munopotentiation and age-retarding etc. Furthermore, RJ
Biomedical Research 2011 Volume 22 Issue 1 functions as an immune-activator including antimicrobial [1] and antitumor [2] activities as well as an inhibitor of stress-related immunodepression [3]. However, the bioac-tive mechanisms of RJ remain poorly understood.
RJ has been known to show many pleiotropic functions [4,5,6,7] in humans, and as a function of them, it is capa-ble of exhibiting potential immunomodulation in mice by stimulating antibody production and immunocompetent cell proliferation [8,9,10]. Therefore, we asked whether RJ could restore the alcohol-induced liver injury in mice. Long-term ingestion of alcohol causes serious hepatic insufficiency, such as aggravation of viral hepatitis, and elicits fatty liver, and fibrosis/cirrhosis. Alcohol abuse and alcoholic liver disease cause serious social problems in many countries. There are several proposed mechanism to explain alcoholic liver injury, such as variations in alcohol metabolism [11], centrilobular hypoxia [12], inflamma-tory cell infiltration and activation[13] [14], antigenic adduct formation [15] etc. there are several new and com-pelling theories concerning alcoholic liver diseases: (1) the aberrance of immune system is induced by attenuated T cell and this is caused by the deterioration of the func-tion of antigen presenting cells [16], (2) and the produc-tion of proinflammatory-cytokines such as TNF-alpha by Kupffer cells driven by enterobacteria endotoxin [17] [18] [19]. An alcoholic hepatic insufficiency mouse model was constructed to investigate the efficacy of RJ in the restora-tion of liver function by focusing on the kinetics of liver nature killer (NK) cells, that have immunostimulatory activity, and humoral immunity associated with cytokine production.
The present study in which RJ was found to significantly ameliorate alcohol-induced liver injury, thus raises specu-lation that RJ may have a beneficial effect in the treatment of alcohol-induced tissue damage. Based on the data of previously published reports and our findings in this study, we hypothesize that the improvement of liver in-jury by RJ may be a balancing action of different RJ in-gredients favoring the host.
Materials and Methods
Animals
C57BL/6 (B6) mice (Japan SLC, Inc.) at the age of 15 weeks, body weight of 22-25g were used in this study. The mice were maintained under specific pathogen-free coons throughout the experiment. All experiments were conducted according to the ethical principles and guide-lines established by the University of the Ryukyus for the care and use of experimental animals.
Generation of alcoholic liver injury mouse model
B6 mice were fed a 5 % ethanol (EtOH) diet (BIO-SERV USA, product #F1258SP) following the instructions of the manufacturer throughout the experiment. The control group of mice was fed a nutritionally adequate liquid diet (BIO-SERV USA, product #F1259) without EtOH. The ethanol concentration of ethanol diets for the experimen-tal group was gradually raised from 0 % to 5 % to accli-matize the mice to ethanol. The 5 % ethanol diet and con-trol diet were provided during the night 12 hours (20:00-8:00), normal diet was provided during daylight (8:00-20:00).
RJ and administration regimens
RJ (RJ) produced by Yamaguchi’s organic bee culture was provided by Japan RJ Co. Ltd., Tokyo. Japan (Lot.201107). The mice were administrated with RJ as de-scribed previously [20]. Briefly, the RJ was diluted in distillated water (DW) and 30 μl (microliter)/mouse of diluted RJ solution containing 2.0 mg of proteins was orally administered for two weeks before ethanol treat-ment was started. The administration regimen was per-formed every day during the experimental period. The control diet and ethanol diet groups were administrated with 30μl PBS as a substitute for RJ.
Quantification of Serum level of transaminase
The mice were anaesthetized with isoflurane and whole blood was collected by cardiac puncture at 4 weeks and 9 weeks. Sera were then separated for quantitative analysis of alanine aminotransferase (ALT) with a Transaminase CII-test kit (Wako Pure Chemical Industries, Osaka, Ja-pan).
Cell preparation
The mice were sacrificed at 4 weeks and 9 weeks follow-ing administration of the 5 % ethanol diet. The mice were anaesthetized with isoflurane and sacrificed by cardiac puncture. The liver and spleen were removed. Hepatic lymphocytes were prepared as previously described [21]. Briefly, the liver was pressed through 200-gauge stainless steel mesh and suspended in Eagle’s MEM supplemented with 5mM Hepes (Nissui Pharmaceutical, Tokyo, Japan) and 2 % FCS. After one washing, the pellet was resus-pended in 35 % Percoll solution containing 100 U/ml he-parin and centrifuged at 2000 rpm for 15 min. The pellet was resuspended in red blood cell (RBC) lysis solution and then washed twice with the medium. Splenocytes were obtained by forcing the spleen through stainless steel mesh. Splenocytes were treated with 0.2 % NaCl solution to remove RBC.
Flow cytometric analysis
The phenotypes of cells were identified by immunofluo-rescence tests with labeled monoclonal antibodies (mAb) [21]. The mAbs used in this study include anti-CD3 (145-2C11), anti-NK1.1 (PK136), (PharMingen, SanDiego, CA). All mAbs labeled with fluorescein isothiocyanate (FITC) or phycocerythrin (PE). Anti-CD16/CD32 (2.4 G2) mAb was added before staining with labeled mAbs to prevent nonspecific binding of mAb. The cells (5 × 105 - 2 × 106/tube) were stained with mAbs and stained cells were analyzed with a FACSCalibur (Becton-Dickinson). Dead cells were excluded by forward scatter, side scatter, and propidium iodide gating.
NK cell cytotoxicity assay
The cytotoxicity assay was performed using the LDH-Cytotoxic Test kit (Wako Pure Chemical Industries, Osa-ka, Japan), according to the manufacturer’s instructions. As the effector cells, liver and spleen mononuclear cells were conventionally-purified, serially diluted with RPMI-1640 Medium without Phenol Red (SIGMA) and mixed with target cells (NK-sensitive YAC-1) in 96-well round-bottomed microculture plate. The plates were brief-ly centrifuged and incubated for 4 h at 37°C. At the end of the culture, 100μl sample of the supernatant was retrieved and lactate dehydrogenase (LDH) released from the target cells was measured colorimetrically with the LDH-Cytotoxic Test kit. Ploy (I:C) which is the ligand of toll like receptor 3 was injected (100 μg/mouse, i.p.) into mice just 12 h before cytotoxicity assay To induce cytotoxicity [21].
Measurement analysis of cytokine levels
Sera were collected from the peripheral blood of mice; levels of various types of cytokines were quantified by the Cytometric Bead Array (CBA) Cytokine kit (Becton-Dickinson).
Statistical analysis
The statistical significance of the data was determined by Student’s t-test using a computer software program (JMP, SAS Institute Inc., Cary, NC). A p value of < 0.05 was considered to be significant.
Results
Influence of RJ on the weight of body and organs
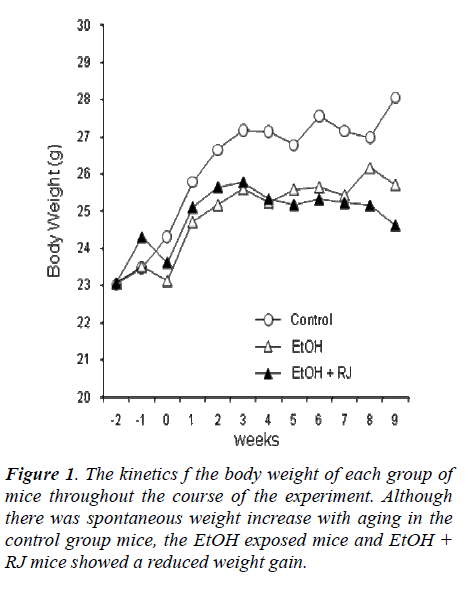
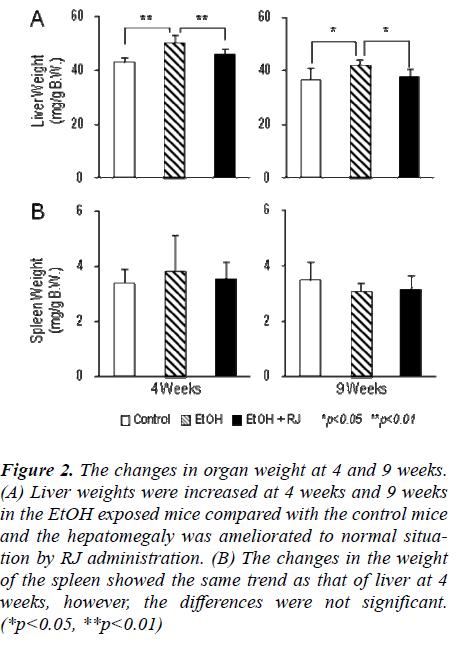
The kinetics of body weights and organ weights of (1) control mice, (2) EtOH-exposed mice and (3) EtOH-exposed plus RJ treated mice were estimated to assess the effect of RJ administration on alcoholic hepatitis. As shown in Figure 1, the mice fed the control diet had in-creased body weight with advancing age. In contrast, tOH-exposed mice with or without RJ administration did not gain significant body weight in comparison to the mice fed the control diet. Although RJ administration did not ameliorate the reduction of body weight in the EtOH-exposed mice (Fig. 1), RJ administration did show a pre-dominant prophylactic effect on hepatomegaly at 4 weeks and 9 weeks (Fig. 2 A). However, RJ administration did not show any prophylactic effect on splenomegaly (Fig. 2 B).
Influence of RJ on hepatic insufficiency caused by al-cohol
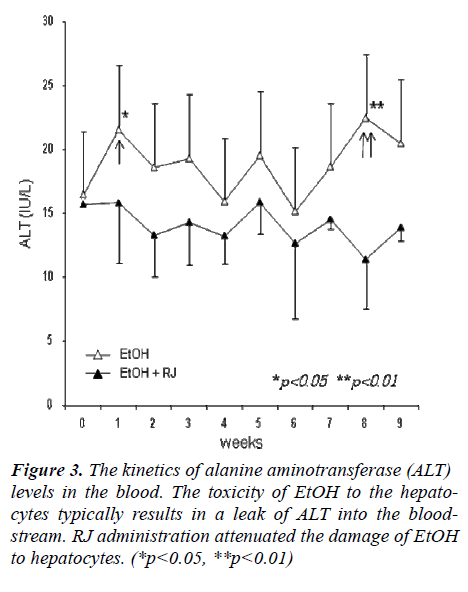
Alcohol consumption produces toxic chemicals like acet-aldehyde which can damage liver cells, followed by an elevation of Alanine transaminase (ALT) levels in the blood. Fluctuations of ALT levels were observed in the blood of EtOH-administrated mice and RJ administrated mice. As shown in Figure 3, the increase in ALT, which was caused by EtOH, was controlled by RJ administra-tion, especially at the early and late stages. The ALT level was elevated sharply at the early stage of EtOH ingestion (Fig. 3, single arrowhead). It is possible that the initial
Figure 3: The changes in organ weight at 4 and 9 weeks. (A) Liver weights were increased at 4 weeks and 9 weeks in the EtOH exposed mice compared with the control mice and the hepatomegaly was ameliorated to normal situa-tion by RJ administration. (B) The changes in the weight of the spleen showed the same trend as that of liver at 4 weeks, however, the differences were not significant. (*p<0.05, **p<0.01)
Influence of RJ on the number of mononuclear cells (MNC) and their subsets
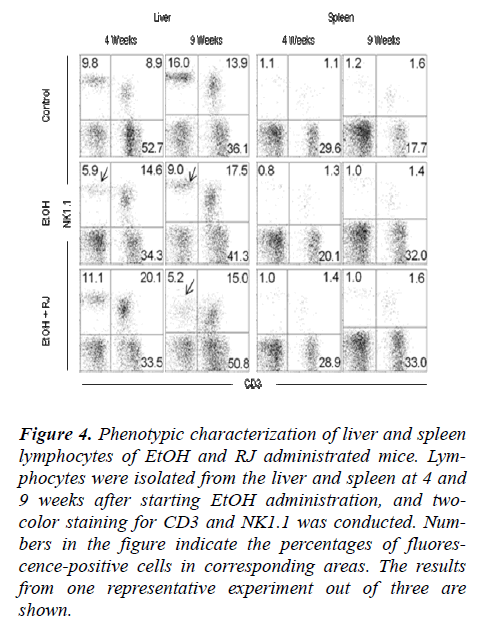
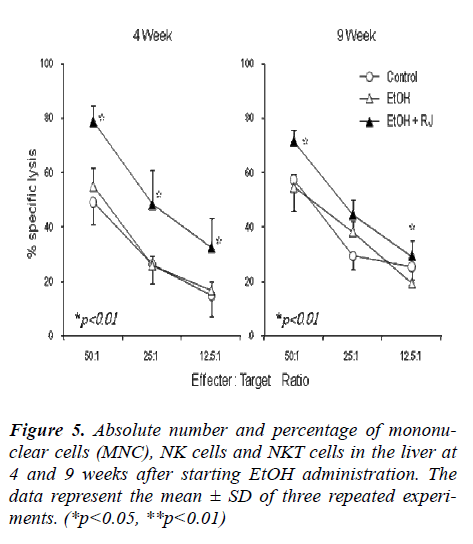
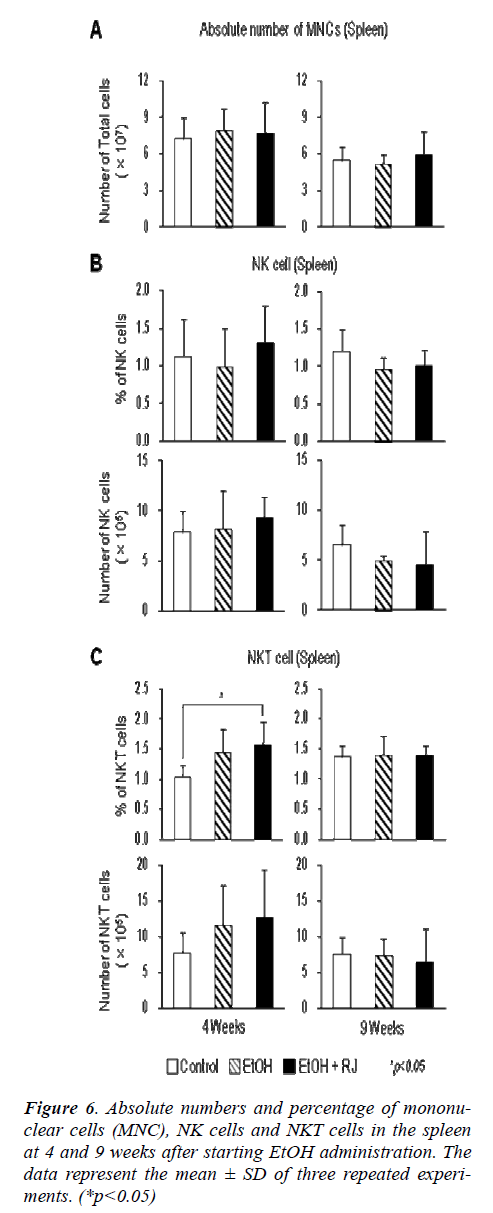
The total MNCs were separated from the liver and spleen of EtOH and RJ treated mice and the phenotypes of MNCs were observed by flow cytometry to evaluate the affect of RJ in the regulation of MNCs. Additionally, the absolute number of MNCs on weeks 4 and 9 after EtOH administration were calculated. The absolute numbers of liver MNCs increased at 9 weeks in the RJ administrated mice in comparison to the control and EtOH adminis-trated mice (Fig. 5A). The percentage of NK (CD3+ NK1.1-) cells decreased at 4 weeks in EtOH administrated mice. However, although there was a prominent decrease in the percentage of NK cells in both the EtOH and RJ administrated mice at 9 weeks (Fig. 4 and Fig. 5B); there was no significant change in the absolute number of NK cells (Fig. 5B). Even though there was no statistical sig-nificance difference in the percentage of NKT (CD3NK1.1) cell fractions (Fig. 5 and Fig. 5C), the absolute number of NKT cells showed a significant increase at 4 weeks in EtOH administrated mice compared with control mice; and at the 9 weeks the absolute number of NKT cells showed a trend to increase in the RJ administrated mice compared with the normal and EtOH administrated mice (Fig. 5C). The absolute number of NKT cells in the MNCs in the spleen significantly increased in the EtOH and EtOH+RJ groups of mice at 4weeks.
Figure 4: Phenotypic characterization of liver and spleen lymphocytes of EtOH and RJ administrated mice. Lym-phocytes were isolated from the liver and spleen at 4 and 9 weeks after starting EtOH administration, and two-color staining for CD3 and NK1.1 was conducted. Num-bers in the figure indicate the percentages of fluores-cence-positive cells in corresponding areas. The results from one representative experiment out of three are shown.
Increase in NK cell cytotoxicity by RJ administration
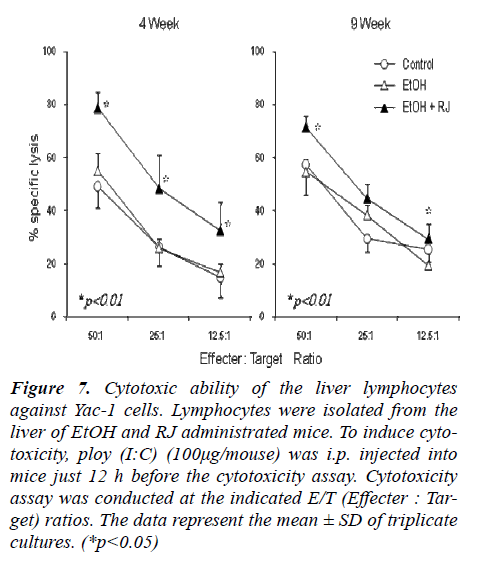
Cytotoxicity against Yac-1 cells (NK cytotoxicity) was examined using liver lymphocytes as effector cells (Fig. 7). Liver lymphocytes were isolated from EtOH- exposed mice and RJ administrated mice. It was observed that NK cytotoxicity was augmented by RJ administration at 4 weeks after EtOH administration compared with the con-trol and EtOH administrated mice. Since NK cytotoxicity is known to be augmented by toll like receptor 3 ligand poly (I:C) [21] [22], this reagent was injected into mice in vivo just one day before sacrifice. In all tested cases, the cytotoxic effects were augmented by the effect of poly (I:C), especially, at 4 weeks after EtOH administration.
Figure 7: Cytotoxic ability of the liver lymphocytes against Yac-1 cells. Lymphocytes were isolated from the liver of EtOH and RJ administrated mice. To induce cyto-toxicity, ploy (I:C) (100μg/mouse) was i.p. injected into mice just 12 h before the cytotoxicity assay. Cytotoxicity assay was conducted at the indicated E/T (Effecter : Tar-get) ratios. The data represent the mean ± SD of triplicate cultures. (*p<0.05)
Influence of RJ on the Th1/Th2 cytokine profiles
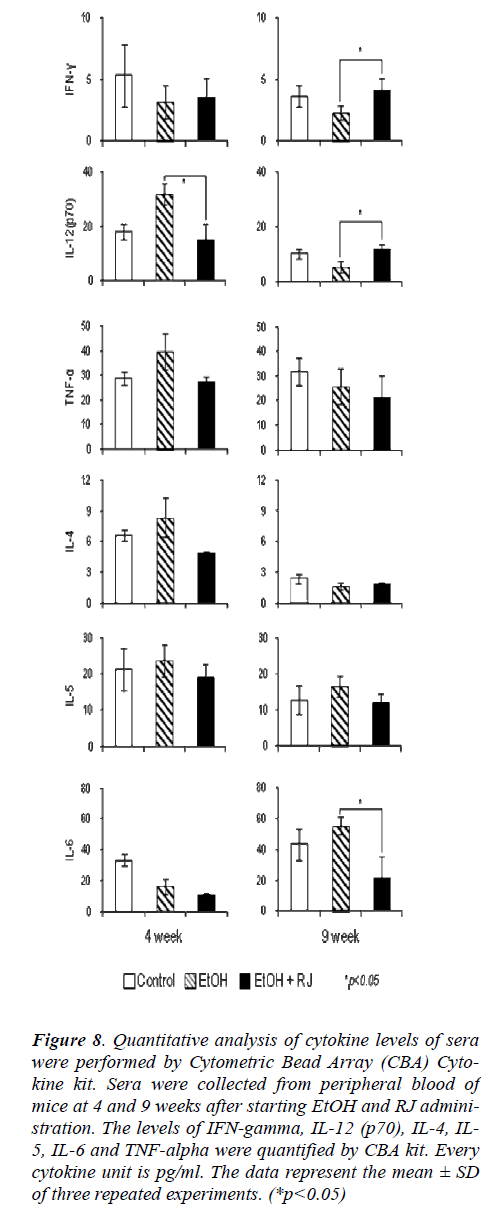
Ethanol stimulus is known to modulate the cytokine lev-els in a variety of tissues including plasma, liver, lung, and brain [23]. The Th1 and Th2 cytokine balance is very important in physiological homeostasis. Th1/Th2 cytokine imbalance induces various physiological disorders. Im-munocytes produce several cytokines to modulate the activation or inhibition of various immunocytes in the autocrine or paracrine fashion. The Th1 and Th2 cytokine profiles of serum were investigated to assess whether RJ could regulate the balance of Th1/Th2 cytokine. As shown in Figure 8, RJ maintained the equilibrium of Th1 and Th2 cytokines by reducing the increased levels of cytokines caused by EtOH stimuli. EtOH toxicity induced an increase in Th1 cytokines such as IL-12 and TNF-alpha at the early stage in EtOH administered mice com-pared with control mice, and the increase was signifi-cantly inhibited by the administration of RJ. In contrast, the production of Th1 cytokines such as IFN-gamma and IL-12 was suppressed by EtOH toxicity at the late stage, and the administration of RJ significantly restored the ability of production of these cytokines. Although the Th2 cytokines such as IL-4, IL-5 and IL-6 showed a tendency to increase in the EtOH-exposed mice, the RJ administra-tion suppressed the excessive secretion of these Th2 cyto-kines.
Figure 8: Quantitative analysis of cytokine levels of sera were performed by Cytometric Bead Array (CBA) Cyto-kine kit. Sera were collected from peripheral blood of mice at 4 and 9 weeks after starting EtOH and RJ admini-stration. The levels of IFN-gamma, IL-12 (p70), IL-4, IL-5, IL-6 and TNF-alpha were quantified by CBA kit. Every cytokine unit is pg/ml. The data represent the mean ± SD of three repeated experiments. (*p<0.05)
Discussion
Alcoholic liver disease is one of the most serious medical consequences of chronic alcohol consumption. Long-term ingestion of alcohol causes serious hepatic insufficiency induces alcoholic hepatitis, fatty liver, and fibro-sis/cirrhosis. Alcoholic hepatic insufficiency is caused by a variety of mechanisms, including the deterioration of T cell function and the production of inflammatory cyto-kines by activated Kupffer cells in the liver. For this rea-son, in the present study we demonstrated the effects of RJ on alcoholic liver injury by focusing on innate-immune responses. The results demonstrated that RJ (1) prevented hepatomegaly, (2) suppressed serum transami-nase levels, (3) induced natural killer cell activation, and (4) caused reduction of IL-4, IL-5 and TNF-alpha in the sera. These results indicate that RJ has the ability to nor-malize the immune system during alcoholic damage. The precise mechanism of how RJ functions in the ameliora-tion of liver injury remains unknown. However, the main-tenance of a well-balanced immune system by RJ was reported previously by our group [3]. Therefore we hy-pothesized that RJ has the immunomodulatory and/or immunoactivating functions in alcoholic hepatic failure in favor of the host.
The functional foods of natural origin are known to have immunoactivating functions, especially, the hyperergasia of NK and NKT cells in the liver received attention. NK and NKT cells are innate immunocytes and these cells function in immunosurveillance. The present study, con-firmed an increasing trend in the absolute number and percentage of NK cells in the liver of RJ administrated mice compared with EtOH ingested mice, although there was no statistical significance. Mice which received oral administration of RJ exhibited a predominant cytotoxic activation of NK cells in the liver of an alcoholic hepatic insufficiency model. It is thought that the activated NK cells may eliminate the hepatocytes damaged by alcohol toxicity and advance the regeneration of hepatocytes. Several papers have demonstrated that alcohol ingestion causes an increase in NKT cell numbers and enhancement of cytotoxic activation of NKT cells in the liver [24]. In our alcohol free-feeding mouse model, the increase of NKT cells was confirmed at the early stage but not at the late stage of alcohol ingestion compared with control diet mice. These findings suggest that there is lack of correla-tion between the increased NKT cell and long-term alco-hol ingestion.
Inflammatory cytokines, such as TNF-alpha and IL-1 which are produced by activated Kupffer cell are involved in the mechanism of alcoholic liver injury [13]. On the other hand, our study showed that IL-6 which is a Th2 cytokine was significantly decreased in the late stage (9 weeks) of RJ administrated mice compared with EtOH ingested mice. Conversely, the Th1 cytokines, especially IFN-gamma and IL-12 were relatively increased in the late stage of RJ administrated mice compared with EtOH ingested mice. The Th1 and Th2 cytokine balance func-tions as a determinant of alcoholic liver injury and TNF-alpha plays a pathological role in the toxicity [25]. It is evident from the current study that TNF-alpha was in-creased in the early stage of EtOH exposure, and the augmentation of TNF-alpha was suppressed by RJ ad-ministration during the whole course of the experiment. This suppression of TNF-alpha production was conducive to inhibition of the alcoholic liver injury and amelioration of hepatic function. The comprehensive survey of various cytokine production patterns in this study shows that RJ has apparent roles in the maintenance of equilibrium of Th1/Th2 cytokines. In brief, RJ restores the aberrant im-mune responses and suppresses the supernumerary re-sponse of immunity during prolonged alcohol exposure.
The detailed mechanism regarding the activity of RJ in immunoregulation has not yet been completely eluci-dated, and controversy remains concerning the indubita-ble efficacy of functional foods. According to the data of previously published reports and our results in this study, it is considered that RJ as the time-honored remedy has the unequivocal capacity of ameliorating effect in alco-holic liver injury. In summary our data indicate that RJ has the capacity to help normalize the immune abnormali-ties associated with alcohol consumption.
Acknowledgement
This work was fully supported by Japan RJ (JRJ) Ltd., Tokyo, Japan.
Disclosures
The authors have no financial conflicts of interest.
Abbreviations used: RJ, Royal Jelly; EtOH, ethanol; ALT, alanine aminotransferase; NK, nature killer; LDH, lactate dehydrogenase; DW, distillated water;
References
- Fujiwara, S., et al., A potent antibacterialprotein in RJ. Purification and determination of the primary structure ofroyalisin. J Biol Chem 1990; 265: 11333-7.
- Bincoletto, C., et al., Effects produced by RJ onhaematopoiesis: relation with host resistance against Ehrlich ascites tumourchallenge. Int Immunopharmacol 2005; 5: 679-88.
- Mannoor, M.K., et al., The efficacy of RJ in therestoration of stress-induced disturbance of lymphocytes and granulocytes.Biomedical Research-India 2008; 19: 69-77.
- Sver, L., et al., A RJ as a new potentialimmunomodulator in rats and mice. Comp Immunol Microbiol Infect Dis 1996; 19:31-8.
- Okamoto, I., et al., Major RJ protein 3 modulatesimmune responses in vitro and in vivo. Life Sci 2003; 73: 2029-45.
- Nagai, T., et al., Antioxidant properties ofenzymatic hydrolysates from RJ. J Med Food 2006; 9: 363-7.
- Narita, Y., et al., RJ stimulates bone formation:physiologic and nutrigenomic studies with mice and cell lines. BiosciBiotechnol Biochem 2006; 70: 2508-14.
- Hidaka, S., et al., RJ prevents osteoporosis inrats: beneficial effects in ovariectomy model and in bone tissue culture model.Evid Based Complement Alternat Med 2006; 3: 339-48.
- Majtan, J., et al., The immunostimulatory effect ofthe recombinant apalbumin 1-major honeybee RJ protein-on TNFalpha release. IntImmunopharmacol 2006; 6: 269-78.
- Stocker, A., et al., Trace and mineral elements inRJ and homeostatic effects. J Trace Elem Med Biol 2005; 19: 183-9.
- Nagy, L.E., Molecular aspects of alcoholmetabolism: transcription factors involved in early ethanol-induced liverinjury. Annu Rev Nutr 2004; 24: 55-78.
- French, S.W., N.C. Benson, and P.S. Sun,Centrilobular liver necrosis induced by hypoxia in chronic ethanol-fed rats.Hepatology 1984; 4: 912-7.
- Uesugi, T., et al., Toll-like receptor 4 isinvolved in the mechanism of early alcohol-induced liver injury in mice.Hepatology 2001; 34: 101-8.
- Tsukamoto, H. and S.C. Lu, Current concepts in thepathogenesis of alcoholic liver injury. FASEB J 2001; 15: 1335-49.
- Dey, A. and A.I. Cederbaum, Alcohol and oxidativeliver injury. Hepatology 2006; 43: S63-74.
- Connolly, M.K., et al., In liver fibrosis,dendritic cells govern hepatic inflammation in mice via TNF-alpha. J ClinInvest 2009; 119: 3213-25.
- Adachi, Y., et al., Inactivation of Kupffer cellsprevents early alcohol-induced liver injury. Hepatology 1994; 20: 453-60.
- Thurman, R.G., II. Alcoholic liver injury involvesactivation of Kupffer cells by endotoxin. Am J Physiol 1998; 275: G605-11.
- Radosavljevic, T., D. Mladenovic, and D. Vucevic,[The role of oxidative stress in alcoholic liver injury]. Med Pregl 2009; 62:547-53.
- Mannoor, M.K., et al., Honeybee RJ inhibitsautoimmunity in SLE-prone NZB x NZW F1 mice. Lupus 2009; 18: 44-52.
- Li, C., et al., Immunopotentiation of NKT cells bylow-protein diet and the suppressive effect on tumor metastasis. Cell Immunol2004; 231: 96-102.
- Minagawa, M., et al., Enforced expression of Bcl-2restores the number of NK cells, but does not rescue the impaired developmentof NKT cells or intraepithelial lymphocytes, in IL-2/IL-15 receptorbeta-chain-deficient mice. J Immunol 2002; 169: 4153-60.
- Crews, F.T., et al., Cytokines and alcohol.Alcohol Clin Exp Res 2006; 30: 720-30.
- Minagawa, M., et al., Activated natural killer Tcells induce liver injury by Fas and tumor necrosis factor-alpha during alcoholconsumption. Gastroenterology 2004; 126: 1387-99.
- Masubuchi, Y., S. Sugiyama, and T. Horie, Th1/Th2cytokine balance as a determinant of acetaminophen-induced liver injury. ChemBiol Interact 2009; 179: 273-9.