Review Article - Biomedical Research (2017) Volume 28, Issue 9
The efficacy of nano-silver and silver sulfadiazine for degree II burn wound: a meta-analysis
Zhufeng Liu1, Anyi Zhou1, Xiaojin Zhang1, Cailing Zhao2, Hongyun Li3 and Liming Gao2*1The First Department of Surgery, Laiwu City People’s Hospital Affiliated to Taishan Medical University, Laiwu, PR China
2Department of Plastic and Burn Surgery, Laiwu City People’s Hospital Affiliated to Taishan Medical University, Laiwu, PR China
3Department of Plastic and Burn Surgery, Shandong Provincial Qianfoshan Hospital, Jinan, PR China
- *Corresponding Author:
- Liming Gao
Department of Plastic and Burn Surgery
Laiwu City People’s Hospital Affiliated to Taishan Medical University
China
Accepted date: January 27, 2017
Abstract
This study is to compare the treatment efficacy between nano-silver and silver sulfadiazine for the degree II burn wound. We searched the Cochrane Library, PubMed, EMbase, Chinese BioMedical Literature database (CBM), Chinese National Knowledge Infrastructure (CNKI) and VIP database from inception to November 2016. The Randomized Controlled Trials (RCTs) comparing the treatment efficacy of nano-silver and silver sulfadiazine for degree II burn was collected. The literature screening, data extraction and assessment of bias were independently screened by two investigators. Seventeen RCTs, involving a total of 1575 patients, were finally included. The overall methodological quality of included studies was good. Compared with silver sulfadiazine, nano-silver significantly decreased the healing time in patients with both the superficial and deep degrees II burn (Mean Difference (MD)=-3.30, 95% CI: -3.88~-2.72, P<0.00001; MD=-3.26, 95% CI: -4.03~-2.49, p<0.00001 respectively). The wound healing rate at 15 days after burn was significantly higher in patients treated with nanosilver compared with that in patients treated with silver sulfadiazine (MD=9.83, 95% CI: 4.02~15.64, P=0.0009). Finally, compared with silver sulfadiazine, nano-silver treatment significantly reduced the incidence of secondary bacterial infection after burn (RR=0.40, 95% CI: 0.27~0.58, p<0.00001) and the visual analog scale of patients (MD=-1.04, 95% CI: -1.34~-0.75, P<0.00001). Our results indicated that nano-silver significantly promote wound healing of degree II burn and reduce the incidence of bacterial infection compared with silver sulfadiazine.
Keywords
Nano-silver, Silver sulfadiazine, Degree II burn, Randomized controlled trials, Meta-analysis
Introduction
The secondary infection, with a very high incidence, is one type of the most serious complications and the main causes of death in burn patients. Infections mostly occur in burn wound especially the deep degree II wound or deeper superficial degree II wound [1]. The occurrence of local vascular thrombosis and serious inflammatory response frequently lead to the inability of systemic administration of antibiotic for the local infection [2]. Therefore, the efficacy of the intravenous antibiotic alone to control bacterial reproduction in the burn wound is not satisfactory. Besides, long-term use of antibiotic is also easy to result in multi-drug resistance and superinfection, which will bring more difficulties to the treatment [3]. Early topical application of bactericidal or antibacterial agents is an effective measure which could effectively protect the burn wound from bacterial infection and ultimately promote the wound healing [4].
Silver-carrying antimicrobial preparation has been used for burn wound as an externally applied agent for several decades. Silver have synergy effect with sulfadiazine in inhibiting bacterial growth [5]. Silver sulfadiazine is one of the traditional silver formulations, which play an important role in inhibiting the molecular transport system, enhancing the stability of DNA and finally decreasing bacterial replication [5]. Besides, silver sulfadiazine exerts antimicrobial effects through destroying the molecular structure of bacteria and increasing the production of inactive and insoluble metabolites [6]. These metabolites further prevent bacterial infection, alleviate local inflammation reaction and ultimately promote wound healing [7]. Nano-silver surgical dressing, produced by attaching 25 nm silver ions to the cotton fiber, has potent antibacterial properties, even without the synergy effect with sulfadiazine. Silver ions are released slowly to combine with anion of the bacterial protein and finally lead to denaturation of bacterial protein [6]. Nano-silver has several advantages compared with silver sulfadiazine. Firstly, the nano-silver has a larger contact area compared with the conventional silver formulation, thus, the requisite amount of silver ions are greatly reduced for the equal amount of bacteria. It is of great significance to improve efficacy and safety of silver therapies. Secondly, nano-silver dressing has the superior permeability and is of advantageous for the drainage of wound, which significantly reduces the risk of infection caused by subcutaneous effusion [7]. Thirdly, the nano-silver greatly facilitates the wound healing through inhibiting the secretion of matrix metalloproteinases in the local site of wound [8]. Finally, nano-silver dressing has an antibacterial or bactericidal effect against a wide spectrum of bacteria including Clostridium perfringens, Staphylococcus aureus, Enterobacter, and Candida albicans [9]. More importantly, nano-silver dressing could be less likely than traditional antibiotics and silver sulfadiazine to spur the development of drug-resistant bacteria [8]. Therefore, it is of great importance to comprehensively evaluate the efficacy of nano-sliver.
We conducted this meta-analysis based on the Cochrane Systematic Review to compare the efficacy of the two treatment regimens in the healing time, healing rate, incidence of secondary bacterial infection and other outcomes in the treatment of degree II burn wound, and to provide relevant evidence for clinical decision-makers in clinical therapy.
Materials and Methods
Inclusion and exclusion criteria
Studies fulfilling the following inclusion criteria were included in this meta-analysis: (1) study type of Randomized Controlled Trials (RCTs); (2) studies including burn patients without restriction of age and gender; (3) patients in intervention group were treated by nano-silver, nano-silver formulations or nano-silver combined with other drugs; while patients in control group were treated by silver sulfadiazine; (4) endpoints included wound healing time, the number of wound healing and the rate of bacterial infection. Following studies were excluded in the final analysis: (1) full text or sufficient data of two groups cannot be obtained; (2) descriptive studies or clinical studies without appropriate control group; (3) literature reviews, case reports or editorials; (4) studies of animal experiments; (5) comparative studies of the clinical efficacy between the nano-silver and other non-silver sulfadiazine agents.
Literature search
We conducted a systematic search of RCTs from English electronic database including Cochrane Library, MEDLINE (PubMed) and EMbase, and Chinese database including Chinese BioMedical literature Database (CBM), Chinese National Knowledge Infrastructure (CNKI) and VIP database from inception to November 2016. The searching words included English terms of “nano crystalline silver” and “burn” and Chinese terms of “nano-silver” and “burn” in the English and Chinese database respectively. A search strategy of combination of random words and subject words was used.
Study selection and methodological assessment
The study design including the double-blinding and randomization were independently assessed by two investigators. According to the bias risk assessment tools recommended by Conchrane Handbook version 5.0 [10], evaluation of methodological quality of studies was conducted and scored as following: 0: No description or improper description and implementation of the randomized or double-blinded methods; or no description of the number and reasons for participants who withdraw or was terminated from the study; 1: description of the randomized or double-blinded methods; or description of the number and reasons for participants who withdraw or was terminated from the study; 2: appropriate application and description of the randomized or double-blinded methods; or proper application of single-blinded clinical research (blinded to the statisticians or efficacy evaluators). A total score of 5 points was classified into grade A ≥ 3 points, grade B=2 points, and grade C ≤ 1 points [10].
Statistical analysis
All meta-analysis were conducted using Review Manager (RevMan) version 5.2 statistical software provided by the Cochrane Collaboration. Counting data and measurement data were analysed using Relative Risk (RR) with 95% Confidence Interval (95% CI) and Mean Difference (MD) with 95% CI respectively for the assessment of drug efficacy. Heterogeneity across the included studies was evaluated by χ2 test and I2- values. If there was no statistical heterogeneity (p>0.1 and I2<50%), fixed effects model was applied; otherwise, if there was statistical heterogeneity (p<0.1 and I2>50%), the sources of heterogeneity was explored to determine whether the random effects model was available in the process of metaanalysis. If that were obviously heterogeneous among the included studies, only description of the features of the studies was demonstrated. P<0.05 was considered as statistically significant.
Results
Literature search and methodological quality assessment
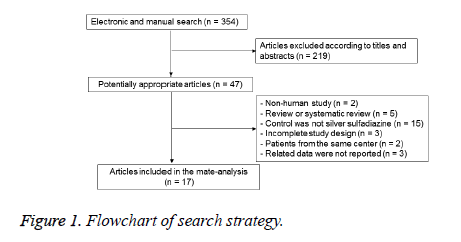
The initial application of search strategy yielded 354 potentially relevant RCTs studies; after removal of duplicated studies and screening the full-text of articles according to inclusion and exclusion criteria, 17 studies [11-27] involving 1575 participants were ultimately included. The flow chart of our search strategy was shown in Figure 1. There were no significant differences among the included studies at baseline. The quality of studies were evaluated and classified into grade A of 4 studies [13,24-26], grade B of 12 studies [11,12,15-23,27] and grade C of 1 studies [14]. Based on the recommendation of Conchrane Handbook, the approach of randomization method were carried out in 8 studies [13,16,19,20,24-27], appropriate allocation concealment were used in 5 studies [13,19,24-26], and the blinding methods of reporting results were used in 8 studies (Table 1) [13,16,19,20,24-27].
| Study | Participants, n (nano-silver/control) | Randomization | Allocation concealment | Blinding | Incompleteness of data | Grade |
|---|---|---|---|---|---|---|
| Li [11] | 48/48 | Unclear | Unclear | Yes | NO | B |
| Chen [12] | 65/46 | Unclear | Unclear | Yes | NO | B |
| Huang [13] | 83/83 | Yes | Yes | Yes | NO | A |
| Zhao [14] | 12-Dec | Unclear | Unclear | Unclear | NO | C |
| Chen [15] | 30/30 | Unclear | Unclear | Yes | NO | B |
| Wang [16] | 46/46 | Yes | Unclear | Yes | NO | B |
| Wang [17] | 47/38 | Unclear | Unclear | Yes | NO | B |
| Zhou [18] | 45/41 | Unclear | Unclear | Yes | NO | B |
| Zhang [19] | 80/80 | Yes | Yes | Yes | NO | B |
| Yang [20] | 100/100 | Yes | Unclear | Yes | NO | B |
| Ou [21] | 30/30 | Unclear | Unclear | Yes | NO | B |
| Zhang [22] | 90/90 | Unclear | Unclear | Yes | NO | B |
| Kang [23] | 40/40 | Unclear | Unclear | Yes | NO | B |
| Muangman [24] | 25/25 | Yes | Yes | Yes | NO | A |
| Varas [25] | 14/14 | Yes | Yes | Yes | NO | A |
| Verbelen [26] | 50/50 | Yes | Yes | Yes | NO | A |
| Brown [27] | 45/44 | Yes | Unclear | Yes | NO | B |
Table 1: Methodological quality assessment of the included studies.
Results of meta-analysis
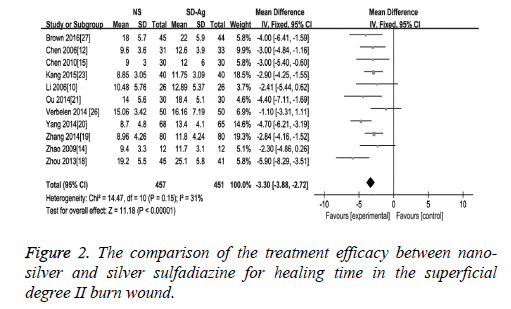
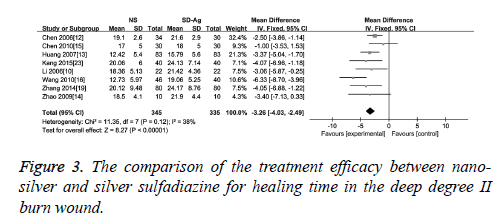
Wound healing time: As an effect indicator and continuous variable, wound healing time was expressed in the form of Mean Difference (MD) in the original articles. The comparison of the treatment efficacy between the groups of nano-silver and silver sulfadiazine for the superficial degree II burn wound was carried out in 10 of 17 included studies. The detection of heterogeneity showed no significant heterogeneity among the studies (I2=31, p=0.25), therefore the fixed effect model was used. The results demonstrated a significant difference of wound healing time between these two groups (MD=-3.30, 95% CI: -3.88~-2.72, P<0.00001), which suggests that nano-silver formulations are more high-efficiency in reducing healing time compared with silver sulfadiazine in patients with superficial degree II burn wound (Figure 2). Eight studies provided healing time data of both nano-silver and silver sulfadiazine for the deep degree II burn wound. As there was no significant heterogeneity among studies (I2=38%, p=0.12), a fixed effect model was used to combine the effective variables in the meta-analysis. The results showed that the healing time of nano-silver treatment group was significantly less than that of silver sulfadiazine treatment for the deep degree II burn wound (MD=-3.26, 95% CI: -4.03, -2.49, p<0.00001) (Figure 3).
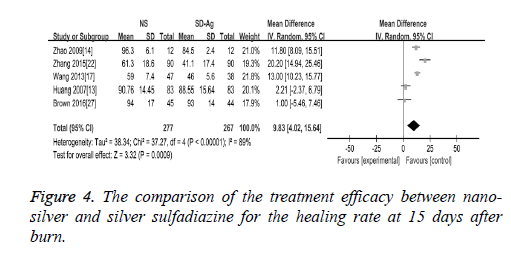
Wound healing rate: There were 5 studies reported the findings of the wound healing rate at 15 days after burn. The random effect model was applied in the meta-analysis as the heterogeneity test demonstrated the presence of the significant heterogeneity across the included studies (I2=89%, P<0.00001). Our findings demonstrated that wound healing rate was significantly higher in nano-silver treatment group than silver sulfadiazine treatment group (MD=9.83, 95% CI: 4.02~15.64, P=0.0009) (Figure 4).
Discussion
Currently, the main drugs for degree II burn are sulfadiazine silver, nano-silver, biological agents, and Chinese herb drugs [28,29]. It is reported that silver sulfadiazine is an effective agent to promote wound healing, reduce wound pain and inhibit bacteria growth at the site of wound, which make it one of the most widely used drugs in burn surgery [30]. However, silver sulfadiazine could cause the damage of normal tissue cells and absorption of a large amount of silver ions, which ultimately result in the dysfunction of liver and kidney [30]. Compared with silver sulfadiazine, nano-silver has unique advantages in the controlling of local infection and promotion of wound healing [31]. However, the differences in other aspects between these two drugs are still unclear.
The incidence of bacterial infection
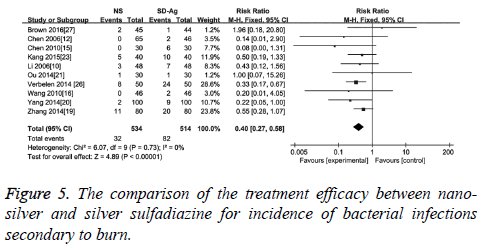
The incidence of bacterial infection secondary to burn was reported in 10 of 17 included studies. No significant heterogeneity among these studies was reported (I2=0, p=0.77). The results of meta-analysis with a fixed effect model suggested that compared with silver sulfadiazine therapy, nano-silver formulations significantly reduced the incidence of bacterial infection secondary to burn wound (RR=0.40, 95% CI: 0.27~0.58, p<0.000 01) (Figure 5).
The visual analog scale (VAS)
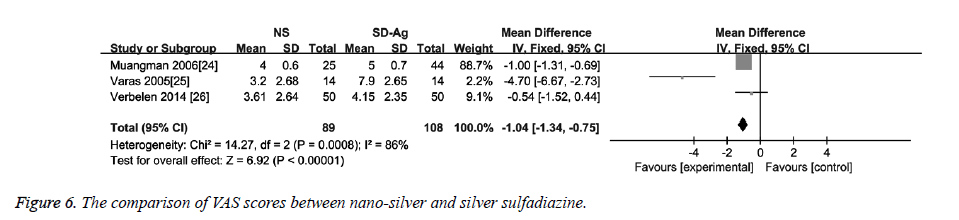
Three studies reported data on VAS score [24-26]. The heterogeneity detection showed significant heterogeneity among the studies (I2=86%, p<0.1). Thus, the random effects model was used. As shown in Figure 6, the VAS score in the nano-sliver group was significantly lower than that in the silver sulfadiazine group (MD=-1.04, 95% CI: -1.34~-0.75, P<0.00001).
In this study, we conducted a meta-analysis to compare the difference of nano-silver and silver sulfadiazine in wound healing rate, wound healing time, incidence of bacterial infection, and VAS score. A total of 17 RCTs involving 1575 patients were analysed. Our findings suggested that, compared with silver sulfadiazine, nano-silver significantly decreased the healing time in patients with degree II burn, including both the superficial and deep degrees. And, nano-silver also significantly increased the wound healing rate at 15 days after burn and reduced the incidence of secondary bacterial infection after burn and the VAS score. These findings may provide relevant evidence for clinical decision-makers in clinical therapy.
However, several limitations in our meta-analysis should be noticed. Firstly, the difference in the patients’ age, causes of burn, forms of nano-silver, and size of wound area will inevitably lead to defects on research consistency and influence the power of the meta-analysis. Secondly, the number of high-quality research articles is limited. Finally, the search strategy was performed with restriction of only English and Chinese languages, and the studies without sufficient details of data were excluded, which inevitably resulted in the selection bias.
In summary, our findings demonstrated that nano-silver significantly reduced the wound healing time, secondary burn infection rate and VAS score. However, the conclusion should be carefully explained and used in clinical practice. A larger sample, multi-centered and randomized controlled trials of high quality should be performed to collect more convincing evidence in the future.
Acknowledgement
The authors thank Chief Ran Huo (from Department of Plastic and Burn Surgery, Shandong Provincial Hospital) for kindly providing helpful advice in the conception, design, results analysis and critical revision in the article.
References
- Zhou F, Qian X. Burn wound infection: causes and countermeasures. Chin J Nosocomiology 2009; 7: 781-783.
- Xu X, Shi C, Zhang B, Cheng S, Song J, Tang Y. Common routes of infection after burn and analysis of its pathogen distribution and drug-resistance. J Dalian Med Univ 2014; 5: 452-455.
- Hassan MM, Kaseb A, Etzel CJ, El-Serag H, Spitz MR. Genetic variation in the PNPLA3 gene and hepatocellular carcinoma in USA: risk and prognosis prediction. Mol Carcinog 2013; 52: 139-147.
- Pang Z, Li H. Antibiotic resistance in multidrug-resistant gram-negative bacteria from burn wards. Practical Clin Med 2015; 1: 31-33.
- Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 2003; 27: 341-353.
- Moiemen NS, Shale E, Drysdale KJ, Smith G, Wilson YT. Acticoat dressings and major burns: systemic silver absorption. Burns 2011; 37: 27-35.
- Fong J, Wood F, Fowler B. A silver coated dressing reduces the incidence of early burn wound cellulitis and associated costs of inpatient treatment: comparative patient care audits. Burns 2005; 31: 562-567.
- Dunn K, Edwards-Jones V. The role of Acticoat with nanocrystalline silver in the management of burns. Burns 2004; 30: 1-9.
- Liu L, Yang M, Lin X, Li Y, Liu C, Yang Y, Yamahara J, Wang J, Li Y. Modulation of hepatic sterol regulatory element- binding protein- 1c- mediated gene expression contributes to Salacia oblonga root- elicited improvement of fructose-induced fatty liver in rats. J Ethno Pharmacol 2013; 150: 1045-1052.
- Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collab 2006; 136.
- Li M, Niu YM, Lin QJ, Wang YX. Oberservation of the protective effect on nanometer silver dressing in burn wound. Zhong Guo Yi Shi Jin Xiu Za Zhi 2006; 15: 11-13.
- Chen J, Han CM, Lin XW, Tang ZJ, Su SJ. Effect of silver nanoparticle dressing on second degree burn wound. Zhonghua Wai Ke Za Zhi 2006; 44: 50-52.
- Huang Y, Li X, Liao Z. A randomized comparative trial between acticoat and SD-Ag in the treatment of residual burn wounds, including safety analysis. Burns 2007; 33: 161-166.
- Zhao ZW, Lei J, Duan P, Hao ZM, Meng YB. Curative effect analysis of 30 cases of burn wound treated with slick nano silver antibacterial gel. Shan Xi Zhi Gong Yi Xue Yuan Xue Bao 2009; 2: 36-37.
- Chen ZJ, Zhao ZW, Duan P. Analysis of 60 cases of burn patients treated with nano silver antibacterial gel and silver sulfadiazine. Shan Xi Yi Yao Za Zhi 2010 9: 874-875.
- Wang CL, Huang YP, Dong YS. Observation of the curing effect of nano silver dressing and sulfadiazine zinc ointment on surface of burn wound. Xian Dai Yi Yuan 2010; 2: 46-47.
- Wang Y, Wang GH, Su WG. Treatment effect of nano-silver antibacterial gel combined with combine recombinant human epidermal growth factor gel in deep 2nd degree burn wound. Zhong Guo Mei Rong Yi Xue 2013; 21: 2098-2099.
- Zhou DD, Li CM, Zhang K, Wang J, Zou PG. Clinical observation of nano-silver antibacterial gel in the treatment of head and face deep second degree burn wounds. He Nan Yi Xue Yan Jiu 2013; 2: 244-245.
- Zhang J, Shi YG, Fu JF. Clinical observation on nano silver dressing for 2° burn wounds. Kun Ming Yi Ke Da Xue Xue Bao 2014; 6: 81-83.
- Yang HG. Comparison of silver sulfadiazine silver ointment and nano silver dressing in treating burn wound. Xian Dai Yang Sheng 2014; 18: 78-79.
- Ou J, Liang XW, Wang XQ. Comparison of clinical effects of nano-silver dressing and sulfadiazine c ream in the treatment of burn wounds. Xian Dai Zhen Duan Yu Zhi Liao 2014; 19: 4386-4388.
- Zhang YS. Treatment of deep 2nd degree burn wounds with nano-silver topical antibacterial gel and ozone. Zhong Guo Yao Ye 2015; 3: 78-79.
- Kang QJ, Hu AG, Liu LP. Clinical application of nano-silver dressing in degree II burn wounds. Jiang Xi Yi Yao 2015; 10: 1039-1041.
- Muangman P, Chuntrasakul C, Silthram S, Suvanchote S, Benjathanung R. Comparison of efficacy of 1% silver sulfadiazine and Acticoat for treatment of partial-thickness burn wounds. J Med Assoc Thai 2006; 89: 953-958.
- Varas RP, OKeeffe T, Namias N. A prospective, randomized trial of acticoat versus silver sulfadiazine in the treatment of partial-thickness burns: which method is less painful? J Burn Care Rehabil 2005; 26: 344-347.
- Verbelen J, Hoeksema H, Heyneman A, Pirayesh A, Monstrey S. Aquacel Ag dressing versus acticoat TM dressing in partial thickness burns: A prospective, randomized, controlled study in 100 patients. Part 1: Burn wound healing. Burns J 2014; 40: 416-427.
- Brown M, Dalziel SR, Herd E, Johnson K, Wong She R, Shepherd M. A randomized controlled study of silver-based burns dressing in a Pediatric Emergency Department. J Burn Care Res 2016; 37: e340-347.
- Zuo YJ, Liao QW, Wang YH. Progress on drug research for treating burn trauma. Hu Nan Zhong Yi Yao Dao Bao 2002; 3: 106-108.
- Lian ZP, Huang LN, Cai H, Zou YT. Research progress of nano-silver dressing in treating burn wounds. Zhong Wai Yi Xue Yan Jiu 2011; 34: 161-162.
- Huang WH. Effect of silver sulfadiazine in treating infection of burn. Lin Chuang He Li Yong Yao Za Zhi 2015; 27: 106-107.
- You CG, Han CM, Wang XG, Sun HF, Hu XL. Antibacterial and toxicity mechanism of silver nanoparticles and its clinical research progress. Zhong Hua Shao Shang Za Zhi 2011; 27: 243.