Research Article - Biomedical Research (2017) Volume 28, Issue 16
The effects of trainings applied with CoQ10 and zinc supplementation on the thyroid hormone metabolism in Soccer players
Metin Polat1*, Yahya Polat1, Taner Akbulut2, Vedat Cinar2 and Irfan Marangoz11School of Physical Education and Sport, Erciyes University, Kayseri, Turkey
2Faculty of Sport Sciences, Firat University, Elazig, Turkey
- *Corresponding Author:
- Metin Polat
School of Physical Education and Sport
Erciyes University, Turkey
Accepted date: July 17, 2017
Abstract
This study aims at examining the effects of zinc and CoQ10 supplementation applied with 8 w trainings on the thyroid hormone metabolism. The study was conducted on 60 voluntary male football players whose average of age is 20.60 ± 3.15 y and average of weight is 66.20 ± 4.74 kg. The participants were divided equally into 4 groups. The groups were constituted in the following way: 1st group: group which is supplied with zinc (Z), 2nd group: group which is supplied with coenzyme Q10 (Q), 3rd group: control group which does only physical exercises (C) and 4th Group: Group which is supplied with zinc and coenzyme Q10 (ZQ). At the first week of the study, TSH, T3 and T4 levels of the participants were measured from the samples which were drawn from the participants at Pre-exercise resting period (PreRP), post-exercise pre-test fatigue (PreTF), and pre-exercise post-test rested (PostTR) and postexercise posttest fatigue (PostTF) after 8 w supplementation period. As a consequence, it was discovered that 8 w trainings led to increases in both rested and post-exercise fatigue TSH levels of only the group supplied with zinc (p<0.05). Considering T3 values, it was discovered that statistically significant differences between the PreTF values and PreTR and PostTR values of the control group and the PreTF values were found to be lower than these two measurements (p<0.05). When CoQ10 supplementation was applied with exercises, differences were observed between the PostTF and PostTR T3 values (p<0.05). As zinc and CoQ10 supplementation were given, post 8 w training T3 values decreased when compared to the pre 8 w training values (p<0.05). Regarding T4 values, it was discovered that PostTF values were lower than the PreTR values of only the group supplied with zinc. The result of this being that, the zinc and CoQ10 supplementation applied with 8 w football trainings were found to be effective on the thyroid hormone metabolism. It is believed that antioxidant supplementation may make significant contributions to the thyroid metabolism functions.
Keywords
Zinc, CoQ10, TSH, T3, T4, Soccer.
Introduction
Zinc is one of the fundamental trace elements which are associated with thyroid hormone metabolism [1]. Zinc has numerous biological functions [2]. It has been reported that thyroid functions are affected adversely in the case of zinc deficiency [3,4]. It has been assured that zinc supplementation, particularly T3, affects thyroid hormone levels positively in individuals who have zinc deficiency [5]. Zinc plays an active role in thyroid hormone metabolism and the process of T4’s being converted into T3 [6]. T3 receptor needs zinc for its biological active structure [2].
CoQ10 [7] that plays a role in bioenergetics is the key element of the ATP formation in oxidative phosphorylation and has a strong lipophilic antioxidant feature, as well [8]. Furthermore, CoQ10 has a role in the oxidation-reduction control of cellular signal pathways [9]. The bioenergetics role of CoQ10 is linked to its antioxidant role. Any situation which leads to increase in oxidative stress reduces the role of CoQ10 in oxidative phosphorylation by increasing its usage as an antioxidant [10]. It has been reported that thyroid hormones have influence over the other hormones by affecting the CoQ10 plasma levels [10].
Thyroid hormones can increase the metabolic rate in almost all tissues. Furthermore, they work for protein synthesis, increasing the number and width mitochondria in several cells; cell’s using glucose rapidly, increasing glycolisis and glyconeogenesis, and increasing fat mobilization [11]. Doing exercise increases the plasma thyroxin concentration yet, when a delay happens between the processes an increase is seen in the TSH concentration and plasma tyroxin concentration during the exercise. Moreover, the thyroxin concentration remains at the same level throughout long-term sub maximal exercise while the triiyodotyronine concentration tends to decrease [11].
Although it is known that thyroid functions show an alteration with exercises, it seems quite difficult to determine these alterations thoroughly. The effect of doing exercises on thyroid hormones depends on gender, datum fitness level, nutritional status, environmental temperature, height, the intensity and type of the exercise and the time measurement is done [12].
The aim of this study is to examine the effects of zinc and CoQ10 supplementation applied with 8 w trainings on the thyroid hormone metabolism.
Materials and Methods
The participants and experimental design
60 voluntary male athletes whose average of age is 20.60 ± 3.15 y and average of weight is 66.20 ± 4.74 kg playing football actively participated in our study. The athletes were equally and randomly divided into 4 groups regarding their age and weight, which is shown in Table 1. At the first week of the study, TSH, T3 and T4 levels of the participants were measured at Pre-exercise Resting Period (PreRP), then, 20 m shuttle running test was applied to create fatigue. The same measurements were taken again after 20 m shuttle run test at post-exercise Pre-Test Fatigue (PreTF). Afterwards, for 8 w, all groups conducted daily 60 min football trainings 4 d a week. Along with the exercises; 1st group was supplied with 22 mg zinc 20-30 min later than dinner once a day, similarly 2nd group was supplied with 100 mg coenzyme Q10, 4th group was supplied with both 22 mg zinc and 100 mg tablet coenzyme Q10 in addition to the normal diet for 8 w, and 3rd Group was not given any supplementation. Fatigue test was performed again 8 w later and TSH, T3 and T4 levels of the participants were measured again before the fatigue test as pre-exercise Post-Test Rested (PostTR) and after the fatigue test as postexercise Post-Test Fatigue (PostTF).
| 1st group: | n:15 | Exercise supplied with zinc |
| 2nd group: | n:15 | Exercise supplied with Q 10 |
| 3rd group: | n:15 | Only exercise |
| 4th group: | n:15 | Exercise supplied with zinc and Q 10 |
Table 1: Research groups.
Identification of the participants’ biochemical parameters
At the beginning and at the end of 8 w program, blood samples were drawn from the athletes both rested and fatigue cases in a total of 4 times. After, the samples were centrifuged at 4000 rpm3 for 10 min, TSH, T3 and T4 levels were determined.
Examination of the blood samples
The blood samples were centrifuged at 4000 rpm3 for 10 minutes and the blood plasma fractionation was done. The measurement of the required parameters was carried out at the central laboratory of Erciyes University Faculty of Medicine. The determination of TSH, T3 and T4 levels were done in a controlled way through Siemens ADVIA 1800 chemistry system device and the findings were calibrated.
Fatigue test
The aim of this test conducted on all groups was to create fatigue in the athletes. 20 m shuttle run test [13] done for that purpose was a multi- stage test and its first stage was comprised of warm-up. The running speed was checked by a tape giving a signal tone. The subjects started to run when they heard the first signal and they reached the other line till the second signal tone. As they heard the second signal, they ran back to the starting point. The race continued with signals. The subjects adjusted their own pace as they were supposed to be at the turnaround spot when they heard the second signal. The frequency of the signals was increased by 0.5 km.h-1 each minute from a starting speed of 8.5 km.h-1. If a subject missed the first signal and caught the second, he continued the test. However, the test was ended if a subject missed both signals. By this way, fatigue was created in the subjects.
The statistical analyses
The statistical analyses were conducted via SPSS 22 (SPSS Inc., Chicago, IL, USA). All measured parameters, mean values and standard errors of all subjects were calculated. The related-samples Friedman’s Two-Way Analysis of Variance by Ranks test was used to identify the differences between the values of the groups obtained through measurements; the significance level was determined as p<0.05.
Results
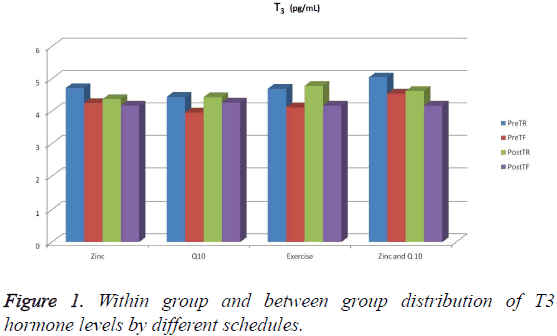
No significant difference was discovered between the pretest rested and pretest fatigue, and posttest rested and posttest fatigue values of the 1st group supplied with zinc (p>0.05). Rather, a significant decrease in the PreTF (Pretest fatigue) levels of the 2nd group supplied with Q 10 in comparison with its PostTR (posttest rested) values (p<0.05) was discovered. Similarly, it was observed that there is a significant decrease in the PreTF (Pretest fatigue) levels of the 3rd group which did only physical exercise in comparison with its PostTR (posttest rested) values (p<0.05). In addition, the PreTF (Pretest fatigue) levels of the same group were found to be significantly lower than the PreTR (pretest rested) levels (p<0.05). And the PostTF (posttest fatigue) levels of the group supplied with zinc and Q10 were significantly lower than the PreTR (pretest rested) levels (p<0.05) (Figure 1 and Table 2).
| Free T3 (pg/ml) | ||||
|---|---|---|---|---|
| Groups | Pretest Rested (PreTR) | Pretest Fatigue (PreTF) | Posttest Rested (PostTR) | Posttest Fatigue (PostTF) |
| Exercise supplied with zinc | 4.71 (4.22-4.86)A | 4.25 (4.04-4.48)AB | 4.37 (4.08-4.63) | 4.17 (4.09-4.47) |
| Exercise supplied with Q 10 | 4.44 (4.18-4.68)abA | 3.96 (3.90-4.17)bA | 4.43 (4.25-4.86)acd | 4.27 (4.11-4.49)bd |
| Only exercise | 4.69 (4.25-4.97)aAB | 4.12 (3.89-4.30)bA | 4.78 (4.36-4.96)b | 4.17 (3.89-4.50)b |
| Exercise supplied with zinc and Q 10 | 5.04 (4.72-5.35)aB | 4.54 (4.22-4.67)abB | 4.62 (3.91-5.08)ab | 4.17 (4.06-4.74)b |
Table 2: Free T3 values of the research groups.
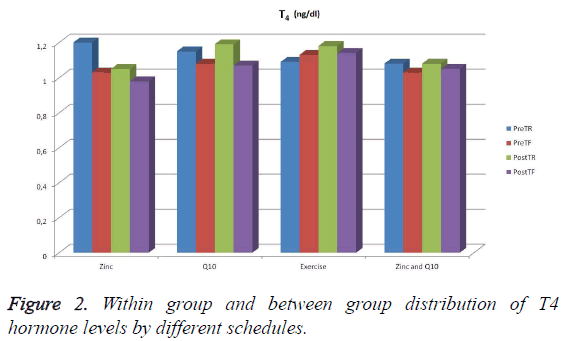
A statistically significant increase in the PreTR (Pretest rested) values of the 1st group supplied with zinc comparison with its PostTF (posttest fatigue) values (p<0.05) was discovered. However, any significant difference was not observed between the pretest rested and pretest fatigue, and posttest rested and posttest fatigue values of the 2nd group supplied with Q 10 (p>0.05). Similarly, no significant differences between the pretest rested and pretest fatigue, and posttest rested and posttest fatigue values of the 3rd group who does only physical exercises (p>0.05) was noticed. And no significant difference was identified between the pretest rested and pretest fatigue, and posttest rested and posttest fatigue values of the 4th group supplied with zinc and Q 10 (p>0.05) (Figure 2 and Table 3).
| Free T4 (ng/dl) | ||||
|---|---|---|---|---|
| Groups | Pretest Rested (PreTR) | Pretest Fatigue (PreTF) | Posttest Rested (PostTR) | Posttest Fatigue (PostTF) |
| Exercise supplied with zinc | 1.20 (1.18-1.29)a | 1.03 (1.01-1.06)ab | 1.05 (0.98-1.11)ab | 0.98 (0.97-1.13)b |
| Exercise supplied with Q 10 | 1.15 (1.22-1.23) | 1.08 (1.03-1.17) | 1.19 (1.08-1.29) | 1.07 (0.96-1.19) |
| Only exercise | 1.09 (1.03-1.22) | 1.13 (1.05-1.23) | 1.18 (1.04-1.22) | 1.14 (0.93-1.22) |
| Exercise supplied with zinc and Q 10 | 1.08 (0.95-1.24) | 1.03 (0.95-1.09) | 1.08 (0.89-1.17) | 1.05 (0.95-1.07) |
Table 3: Free T4 values of the research groups.
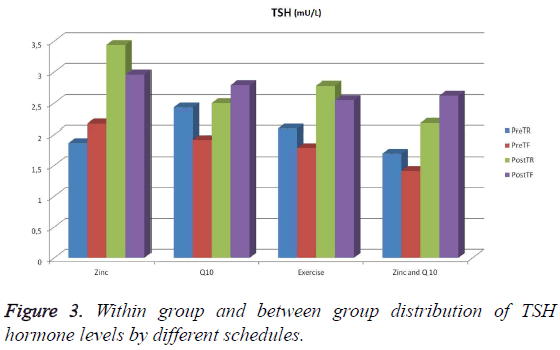
A statistically significant increase in the PostTR (Posttest rested) values of the 1st group supplied with zinc in comparison with its PreTR (pretest rested) values (p<0.05) was observed. Also, a statistically significant increase was noticed between in the PostTF (posttest fatigue) values of the same group in comparison with its PreTR (pretest rested) values (p<0.05). However, no significant difference was observed between the pretest rested and pretest fatigue, and posttest rested and posttest fatigue values of the 2nd group supplied with Q 10 (p>0.05). Similarly, it was not obvious that there was any change between the pretest rested and pretest fatigue, and posttest rested and posttest fatigue values of the 3rd group who does only physical exercises (p>0.05). Also, no significant difference was observed between the pretest rested and pretest fatigue, and posttest rested and posttest fatigue values of the 4th group supplied with zinc and Q 10 (p>0.05) (Figure 3 and Table 4).
| TSH (mU/L) | ||||
|---|---|---|---|---|
| Groups | Pretest Rested (PreTR) | Pretest Fatigue (PreTF) | Posttest Rested (PostTR) | Posttest Fatigue (PostTF) |
| Exercise supplied with zinc | 1.86 (0.64-2.36)aA | 2.17 (1.48-2.38)ac | 3.44 (1.84-4.96)c | 2.96 (2.49-4.01)dc |
| Exercise supplied with Q 10 | 2.43 (1.85-3.28)B | 1.91 (1.74-3.19) | 2.50 (2.47-3.77) | 2.79 (2.11-3.22) |
| Only exercise | 2.09 (1.81-3.46)AB | 1.78 (1.42-2.46) | 2.78 (2.36-3.71) | 2.55 (1.94-2.84) |
| Exercise supplied with zinc and Q 10 | 1.69 (1.56-2.57)AB | 1.41 (1.17-2.68) | 2.18 (1.63-3.21) | 2.62 (1.64-3.19) |
Table 4: TSH values of the research groups.
Discussion
This study aims at examining the effects of the zinc, Q10 and zinc+Q10 supplementation applied four times a week during 8 w along with daily 60 min football trainings on the TSH, T3 and T4 hormone metabolism, the participants’ TSH, T3 and T4 levels were examined through the blood samples drawn 4 times from them to analyse their pretest rested and fatigue and posttest rested and fatigue levels. Considering the differences observed in consequence of analyzing the blood samples, 8 w zinc and Q10 supplementation constituted significant influences on the thyroid hormone metabolism.
When the effect of doing exercises on TSH was examined in literature, different results were obtained. In a study, recreational athletes did bicycle ergometer exercises regularly for 6 w and an increase was observed in TSH and the hormones stimulated by TSH at the end of 6 w [14]. Furthermore, at the end of one-month of aerobic program conducted with 6 healthy women, no change was seen in TSH, T4, T3 and rT3 values of the participants [15]. In the study which examined the effects of 3 d exercise on the soldiers at the North Pole, it was observed that there is a decrease in the participants’ TSH values, and it took more than 48 h until the values returned to the normal level [16]. In our study, we did not identify s any significant change in the pre and post exercise TSH levels of the control group. However, it was observed that post-test rested and post-test fatigue TSH values of the group supplied with zinc were higher than their pretest rested values. The chronic effect of doing exercise on TSH showed a significant increase with zinc supplementation. Zinc has several functions in human organism including endocrine secretion such as thyroid hormones and insulin [17]. Zinc is essential in conversion of Thyroxin (T4) into Triiodothyronine (T3) [18]. It has been discovered that zinc and thyroid hormones are crucial for continuity of the physical performance and energy metabolism in athletes [19]. However, maximal aerobic exercises affect the plasma zinc concentration [20] and thyroid hormone levels in circulatory systems [21] of athletes. It was indicated that zinc deficiency affects thyroid hormone metabolism adversely [22]. Zinc supplementation led to an increase in rested and post-exhausting exercise thyroid hormone levels of the elite wrestlers [23]. In that study, the chronic effect of doing exercise on TSH increased significantly with zinc supplementation in parallel with the findings in literature.
Any significant change was not observed in the pre and postexercise TSH values of the group supplied with CoQ10. In literature, there are studies which state that there are meaningful correlation between thyroid hormones and plasma CoQ10 levels [24,25]. However, it has been proven that hyperthyroidism is associated with the CoQ10 [24] levels in circulatory system. Furthermore, it was discovered that an increase in the CoQ10 level in the case of hyperthyroidism’s turning into hypothyroidim [26]. The reasons of decrease observed in the CoQ10 level while thyroid hormone levels increase are reported to be CoQ10’s running against tyrosine which is a substrate substance for thyroxin synthesis, increase in CoQ10 usage owing to the increase in metabolic necessity, increase in degradation, decrease in the levels of carriers in serum, decrease in very low density lipoproteins secreted by livers in the case of hyperthyroidism [10]. CoQ10 has an antioxidant feature and has a role in bioenergetics [10]. It is possible that the role of CoQ10 in energy generation may lead to decrease in CoQ10 levels during exercise. In our study, no difference was observed in pre and post-exercise TSH hormone levels of the groups supplied with CoQ10 and CoQ10+zinc, which may result from the aforementioned reasons.
Also in our study, as the T3 values of the 3rd group which was not given any supplementation were examined, post-training T3 values at the end of the 1st week were found to be significantly lower than both the level before the participants began trainings and rested T3 values at the beginning of 8th week. This demonstrates that T3 levels decrease while the athletes feel tired after trainings. T3 and T4 are important in the metabolic rate determination and enhancement of the other hormones’ efficiency. Without T3, epinephrine has a little effect on the mobilization of free lipid acids from adipose tissues. Throughout exercise, an increase is seen in free T3 levels because of the changes in adherence characteristics of carrier protein. Nonetheless, T3 and T4 are suspended from plasma by tissues in larger amounts during exercise than the amounts suspended in resting period. TSH secretion from anterior pituitary increases in order to stimulate T3 and T4 secretion from thyroid gland [27]. In our study, post-exercise fatigue T3 levels were found to be lower than the rested values, which probably results from the increase in T3 and T4 usage to provide the energy need of tissues during exercise as mentioned above.
In literature, there are different findings on the chronic effects of exercise on the thyroid functions. In the study of Tremblay et al. [28] conducted on the monozygotic twins, energy intakes were stabilized and it was observed as significant decreases in FT3 and TT3 levels and statistically insignificant decrease in TT4 at the end of the 93 d endurance training in parallel with our findings. In another study, recreational athletes did bicycle ergometer exercises regularly for 6 w and an increase was observed in TSH and the hormones stimulated by TSH at the end of 6 w [14]. Furthermore, at the end of one-month aerobic program conducted with 6 healthy women, no difference was seen in TSH, T4, T3 and rT3 values of the participants [15]. In another study, the participants were given oral liothyronine sodium, and a significant increase was reported in T3 levels of the endurance group [12].
On the other hand, decreases were seen in fatigue T3 values of the groups supplied with CoQ10 and CoQ10+zinc. In addition, posttest fatigue T3 values of the group supplied with CoQ10+zinc were found to be lower than their pretest rested T3 values. Owing to the fact that CoQ10 has a role in energy generation, it was reported that there were negative correlation between thyroid hormones and plasma CoQ10 levels during exercise in certain studies [24,25]. In that study, it is thought that CoQ10 supplementation which causes T3 hormone to decrease during exercise results from the aforementioned reasons.
As T4 hormone results were examined, posttest fatigue values of the group supplied with zinc were found to be lower than the pretest rested values. That finding demonstrates that zinc supplementation decreases the T4 values when the participants were fatigued owing to the chronic effect of trainings. It has been noticed that certain elements in the synthesis and metabolism of thyroid gland and zinc becomes crucial between these elements [29]. Zinc has a role in thyroid hormone metabolism, as well as a role in conversion of T4 into T3 [6]. It was observed that zinc deficiency affects thyroid hormone metabolism adversely [22]. It has been assured that zinc supplementation affects thyroid hormone levels positively in individuals who have zinc deficiency [5]. Morley et al. [30] stated that zinc deficiency caused decreases in T3 and T4 hormones. Gupta et al. [31] reported that zinc deficiency led to decreases in thyroid hormones. It is discovered that there are contradictory findings regarding post-exercise T4 values. Zinc supplementation led to increase in rested and post-exhausting exercise thyroid hormone levels of the elite wrestlers [23]. In another study, it was reported that zinc supplementation had no effect on thyroid hormone levels of cyclists. In another study conducted on physically active women, it was reported T4 levels of the participants increased after they took daily 26.4 mg zinc for 2 months [5]. Marques et al. [32] stated that zinc supplementation (22 mg/d) increased the plasma zinc levels of the elite cyclists who had low plasma zinc values; however, it did not affect the thyroid hormones. In another study conducted on female marathoners and thyroid functions, T3 and T4 levels tended to decrease with weekly 48 km trainings. On the contrary, these hormone levels increased when the training distance was raised to 80 km. It was reported that changes in body composition with high volume trainings might contribute to these changes in women’s thyroid functions which are induced by exercises [33].
Conclusion
According to our findings, after the trainings done with zinc supplementation, the values of the participants were found to be low only when they were fatigued after trainings, but no changes were observed while they rested.
Consequently, the zinc and CoQ10 supplementation which were given individually or collectively to them, and which was applied with trainings 4 d a week for 8 w, made positive contributions to the thyroid hormone metabolism of young football players; therefore, it is thought that antioxidant supplementation may make significant contributions to the football players.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- Prasad AS. Clinical, biochemical and nutritional aspects of zinc. Ann Rev Pharmmacol Toxicol 1982; 20: 267-270.
- Pathak R, Dhawan D, Pathak A. Effect of zinc supplementation on the status of thyroid hormones and Na, K, and Ca levels in blood following ethanol feeding. Biol Trace Elem Res 2011; 140: 208-214.
- Oliver JW, Sachan DS, Su P, Applehans FM. Effects of zinc deficiency on thyroid function. Drug Nutr Interact 1987; 5: 113-124.
- Lukaski HC, Hall CB, Marchello MJ. Impaired thyroid hormone status and thermoregulation during cold exposure of zinc-deficient rats. Horm Metab Res 1992; 24: 363-366.
- Maxwell C, Volpe SL. Effect of zinc supplementation on thyroid hormone function. A case study of two college females. Ann Nutr Metab 2007; 51: 188-194.
- Nishiyama S, Futagoishi-Suginohara Y, Matsukura M, Nakamura T, Higashi A, Shinohara M, Matsuda I. Zinc supplementation alters thyroid hormone metabolism in disabled patients with zinc deficiency. J Am Coll Nutr 1994; 13: 62-67.
- Littarru GP. The mitochondrion: a main energy plant. Energy Defence CESI Roma Italy 1994; 14-21.
- Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr 2001; 20: 591-598.
- Kaltschmidt B, Sparna T, Kaltschmidt C. Activation of NF-κB by reactive oxygen intermediates in the nervous system. Antioxid Red Sig 1999; 1: 129-144.
- Mancini A, Festa R, Raimondo S, Pontecorvi A, Littarru GP. Hormonal influence on coenzyme Q10 levels in blood plasma. Int J Mol Sci 2011; 12: 9216-9225.
- Kenney WL, Wilmore JH, Costill DL. Physiology of sport and exercise. Human Kinetics (6th Edn.) 2015.
- Bernet VJ, Wartofsky L. Thyroid function and exercise. Contemp Endocrinol Sports Endocrinol 2000.
- Leger LA, Mercier D, Gadoury C, Lambert J. The multistage 20 metre shuttle run test for aerobic fitness. J Sports Sci 1988; 6: 93-101.
- Lehmann M, Knizia K, Gastmann U, Petersen KG, Khalaf AN, Bauer S, Keul J. Influence of 6-week, 6 days per week, training on pituitary function in recreational athletes. Br J Sports Med 1993; 27: 186-192.
- Caron PJ, Sopko G, Stolk JM, Jacobs DR, Nisula BC. Effect of physical conditioning measures on thyroid hormone action. Horm Metab Res 1986; 18: 206-208.
- Hackney AC, Hodgdon JA, Hesslink JR, Trygg K. Thyroid hormone responses to military winter exercises in the Arctic region. Arctic Med Res 1995; 54: 82-90.
- Dibley MJ. Zinc. Present knowledge in nutrition. ILSI Washington (DC) 2001; 329-343.
- Danforth E, Burger AG. The impact of nutrition on thyroid hormone physiology and action. Annu Rev Nutr 1989; 9: 201-227.
- Sonksen PH. Insulin, growth hormone and sport. J Endocrinol 2001; 170: 13-25.
- Koury JC, Oliveira AV, Portella ES. Zinc and copper biochemical indices of antioxidant status in elite athletes of different modalities. Int J Sport Nutr Exerc Metabol 2004; 14: 358-372.
- Ciloglu F, Peker I, Pehlivan A, Karacabey K, Ilhan N. Exercise intensity and its effects on thyroid hormones. Neuro Endocrinol Lett 2005; 26: 830-834.
- Kralik A, Eder K, Kirchgessner M. Influence of zinc and selenium deficiency on parameters relating to thyroid hormone metabolism. Horm Metab Res 1996; 28: 223-226.
- Kilic M, Baltaci AK, Gunay M, Gokbel H, Okudan N, Cicioglu I. The effect of exhaustion exercise on thyroid hormones and testosterone levels of elite athletes receiving oral zinc. Neuro Endocrinol Lett 2006; 27: 247-252.
- Mancini A, Conte G, De Marinis L, Oradei A, Littarru GP. Thyroid hormone and oxidative metabolism: coenzyme Q10 in thyroids disease. Coenzyme Q Biol Med 1993; 1: 25-34.
- Mancini A, Marinis L, Calabro F, Sciuto R, Oradei A, Lippa S, Barbarino A. Evaluation of metabolic status in amiodarone-induced thyroid disorders: Plasma coenzyme Q10 determination. J Endocrinol Investig 1989; 12: 511-516.
- Pandolfi C, Ferrari D, Stanic I, Pellegrini L. Circulating levels of CoQ10 in hypo- and hyperthyroidism. Minerva Endocrinol 1994; 19: 139-142.
- Powers SK, Howley ET. Exercise physiology: theory and application to fitness and performance. McGraw-Hill Higher Education (9 Edn.) 2014.
- Tremblay A, Poehlman ET, Despres JP, Theriault G, Danforth E, Bouchard C. Endurance training with constant energy intake in identical twins: changes over time in energy expenditure and related hormones. Metabol 1997; 46: 499-503.
- Arthur JR, Beckett GJ. Thyroid function. Br Med Bull 1999; 55: 658-668.
- Morley JE, Gordon J, Hershman JM. Zinc deficiency, chronic starvation, and hypothalamic-pituitary-thyroid function. Am J Clin Nutr 1980; 33: 1767-1770.
- Gupta RP, Verma PC, Garg SL. Effect of experimental zinc deficiency on thyroid gland in guinea-pigs. Ann Nutr Metab 1997; 41: 376-381.
- Marques LFJ, Donangelo CM, Franco JG, Pires L, Luna AS, Casimiro-Lopes G, Koury JC. Plasma zinc, copper, and serum thyroid hormones and insulin levels after zinc supplementation followed by placebo in competitive athletes. Biol Trace Element Res 2011; 142: 415-423.
- McArdle WD, Katch FI, Katch VL. Essentials of exercise physiology. Lippincott Williams and Wilkins (4th Edn.) 2010.