Research Article - Biomedical Research (2017) Volume 28, Issue 11
The effects of TNF-α and anti-TNF-αMcAb in myocardial injury of rats with traumatic hemorrhagic shock
Zhaolei Qiu, Zhenjie Wang*, Feng Cheng, Chuanming Zheng, Lei Li, Hai Jiang and Zhaohui Du
Department of Emergency Surgery, the First Affiliated Hospital of Bengbu Medical College, 87 Changhuai Road, Bengbu, Anhui, PR China
- *Corresponding Author:
- Zhenjie Wang
Department of Emergency Surgery
The First Affiliated Hospital of Bengbu Medical College, PR China
Accepted on April 6, 2017
Abstract
Objective: To study the effects of Tumor Necrosis Factor-α (TNF-α) and anti-TNF-α Monoclonal Antibody (McAb) in myocardial injury of rats with traumatic hemorrhagic shock.
Methods: Twenty-four adult male Sprague-Dawley (SD) rats were randomly divided into the control group (A1 group), lactate Ringer's solution resuscitation group (A2 group) and anti-TNF-αMcAb resuscitation group (A3 group). A knife was used to cut about 5 cm in the middle of abdomen in rats of the A2 and A3 groups. Animal models of traumatic hemorrhagic shock were established through the femoral artery bleeding. The A2 group was treated with lactate Ringer's solution resuscitation, A3 group was treated with lactate Ringer's solution resuscitation containing anti-tumor necrosis factor-α monoclonal antibody (3 mg•kg-1), while the A1 group was under the same conditions without blood loss and trauma. The contents of Superoxide Dismutase (SOD) and Malondialdehyde (MDA) in myocardium were measured. The levels of serum TNF-α and CK-MB were detected in every group. The pathological changes of myocardium were observed by light microscopy.
Results: The levels of serum TNF-α, CK-MB and the content of MDA in myocardium were increased in A2 and A3 groups comparing with A1 group (P<0.05), while the content of SOD was decreased (P<0.05). The levels of TNF-α (106.85 ± 14.65 pg/ml), CK-MB (535.3 ± 96.7 U/L) and the content of MDA (4.403 ± 0.979 nmol/mgprot) in myocardium in A3 group were much lower than those in the A2 group (P<0.05), but the content of SOD (36.125 ± 2.558 U/mgprot) was increased compared with the A2 group (SOD 28.200 ± 3.752 U/mgprot) (P<0.05). The changes of myocardium histopathology were minor in the A3 group while the A1 group had no manifest change.
Conclusions: TNF-α is an important media in myocardial injury induced by traumatic hemorrhagic shock. Anti-tumor necrosis factor-α monoclonal antibody can reduce myocardial injury, the mechanism perhaps antagonistic activity of TNF-α.
Keywords
Anti-TNF-α monoclonal antibody, Tumor necrosis factor-α, Trauma, Hemorrhagic shock
Introduction
Traumatic Hemorrhagic Shock (THS) is a common severe disease in emergency treatment, featuring heavy injuries and rapid changes which can lead to cardiac arrest and other vital organ damage [1]. Medical staff has been in continuous exploration on how to reduce traumatic hemorrhagic shock damage to vital organs, maintain vital organ function, and improve the shock effect of the treatment, however studies are still need to give more clinical and experience data in this field.
Studies have showed that Tumor Necrosis Factor-α (TNF-α) plays an important role in the development of shock caused by many factors, such as zymosan-induced shock [2] and endotoxic shock [3]. And anti-TNF-α Monoclonal Antibody (McAb) is also proved to be effective in protection against shock [4,5]. Though there are numerous studies on this topic, more clinical and experimental data is still helpful in further reveal the mechanism of THS.
The present study aimed to investigate the effects of TNF-α and its monoclonal antibody on myocardial injury in SD rat with THS through different fluid resuscitation in the SD rat THS model.
Materials and Methods
Animals
Clean grade adult male SD rats weighing 260 to 300 g provided by the Experimental Animal Center of Zhejiang Province. Twenty-four rats were randomly divided into three groups with 8 rats in each group: the control group (A1 group), Ringer's lactate solution resuscitation group (A2 group) and anti-TNF-αMcAb resuscitation group (A3 group).
Establishment of animal models
Clean grade adult male SD rats were not fasted before surgery, and given free access to water. Skin preparation was performed on the neck and left groin region. Then rats were anesthetized by an intraperitoneal injection of 10% urethane (1 ml/100 g). After obtaining satisfactory anesthesia, rats were fixed on operating table. After povidone-iodine disinfection, the right external jugular vein was connected to an infusion pump. An incision which was about 5 cm in length was made in the rat abdomen, creating body injuries in rats. Before catheterization, the catheter was rinsed with heparin (25 U). An animal model of hemorrhagic shock was made by bleeding via left femoral artery catheterization. Carotid arteries were used for connecting the biological signal acquisition system (Nanjing Meiyi Co., Ltd., Model: MedLed). Blood pressure was checked. Through the femoral artery bleeding, blood pressure was lowered to the mean arterial pressure of 40 mmHg within 10 min. The blood pressure was maintained for 60 min.
Shock resuscitation
After establishment of hemorrhagic shock animal models, the A2 group underwent resuscitation using Ringer's lactate solution within 30 min (4 times as much as the amount of blood loss). The A3 group underwent resuscitation using Ringer's lactate solution containing anti-tumor necrosis factor- α monoclonal antibody (3 mg•kg-1) within 30 min (4 times as much as the amount of blood loss), increasing mean arterial pressure to 90 mmHg. Rats in the A1 group without blood loss and trauma were anesthetized and catheterized under the same conditions, and were observed for 90 min.
Sample collection
Rats were sacrificed 2 h after the end of anti-shock resuscitation, and blood was collected via abdominal aorta. Serum was preserved in a -70°C refrigerator for the sake of detecting TNF-α and creatine kinase (CK-MB) levels. Two myocardium tissues of left ventricular were selected. One tissue was washed with physiological saline, and then stored in a -20°C refrigerator. The other tissue was prepared for pathological examination.
Detection of indexes
Level of serum TNF-α was determined by ABC-ELISA method (double-antibody sandwich enzyme-linked immunosorbent assay) as described elsewhere [6]. CK-MB level was detected using an automatic biochemical analyser monitor. Histopathological examination of myocardial tissue (light microscopy) was provided by the Pathology Department in the first affiliated hospital of Bengbu medical college. Malondialdehyde (MDA) was determined by thiobarbituric acid method [7] and Superoxide Dismutase (SOD) was determined by hydroxylamine method [8], kits were purchased from Nanjing Jiancheng Bioengineering Institute.
Statistical methods
The measurement data was expressed by mean ± SD. Independent continuous variables were compared using the t-test. When P-value was less than 0.05 it was considered to be statistically significant. All calculations were made using SPSS 18.0.
Results
TNF-α and CK-MB levels in SD rats
Comparisons of TNF-α and CK-MB levels in SD rats in each group was shown in Table 1. Results showed that TNF-α and CK-MB levels in the serum in the A2 and A3 groups were higher than those in A1 group (P<0.05). However, TNF-α and CK-MB levels in the serum in the A3 groups were lower compared with the A2 group (P<0.05).
| Groups | TNF-α (pg/mL) | CK-MB(U/L) |
|---|---|---|
| A1 Group | 26.1 ± 7.2 | 274.0 ± 28.3 |
| A2 Group | 193.1 ± 20.3** | 727.0 ± 97.3** |
| A3 Group | 106.9 ± 14.7##** | 535.3 ± 96.7##** |
| The q test: compared with the A1 group, **P<0.05; compared with the A2 group, ##P<0.05 | ||
Table 1. TNF-α and CK-MB levels in SD rats (͞x ± S).
The levels of SOD and MDA in myocardium SD rats in each group
MDA levels in myocardial tissues of rats in the A2 and A3 were higher compared with A1 (P<0.05) while SOD levels decreased (P<0.05). MDA levels in the A3 group decreased compared with the A2 group (P<0.05), while SOD levels increased (P<0.05) (Table 2).
| Groups | SOD (U/mgprot) | MDA (nmol/mgprot) |
|---|---|---|
| A1 Group | 41.838 ± 4.563 | 1.079 ± 0.435 |
| A2 Group | 28.200 ± 3.752** | 6.041 ± 1.055** |
| A3 Group | 36.125 ± 2.558##** | 4.403 ± 0.979##** |
| The q test: compared with the A1 group, **P<0.05; and compared with the A2 group, ##P<0.05 | ||
Table 2. Comparisons among MDA and SOD levels in myocardium SD rats in each group (͞x ± S).
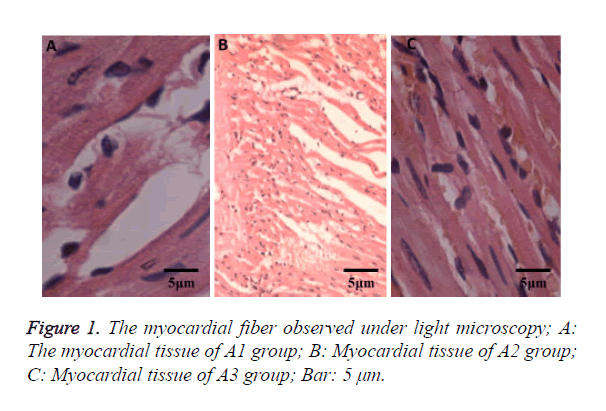
Histopathological examination of myocardial tissue
Cardiomyocytes showed cloudy swelling in SD rats from the A2 group with inflammatory cell infiltration, thinner myocardial fibers and myocardial fiber necrosis. Compared with the A2 group, the myocardial injury in the A3 group was lighter without significant myocardial fiber necrosis (Figure 1). There were no significant changes in myocardial tissues in the A1 group.
Discussion
Trauma [9] refers to the damage of the integrity of body which is caused by external forces and other factors, and ranks as No. 5 killer in China. In 2012, up to 500 million deaths worldwide were resulted from trauma [10]. It is estimated that 40 percent of patients with trauma will die from Hemorrhagic Shock (HS) in 2020 [11]. Early symptom of THS is still hypovolemic shock. Timely infusion and restoring blood volume of the body are the basic life-saving measures [12]. THS is a state characterized by microcirculation disorder. Rapid loss of blood volume which occurred after body injuries causes hemodynamic instability, inadequate blood perfusion, reduction in blood and oxygen supplies as well as ischemia and hypoxia, dysfunction of tissues and cells, eventually leading to death [13].
THS can cause myocardial damage. In this experiment, we studied the effects of TNF-α and its monoclonal antibodies on myocardial injury in SD rats with THS using SD rat THS models which were established by abdominal incision in SD rats combined with bloodletting. This study will provide the basis for clinical treatment.
A study have found that when THS occur, it mainly leads to decline in the body's effective circulating blood volume, internal environment disorder, tissue ischemia, hypoxia, especially intestinal ischemia, severe hypoxia, intestinal function failure as well as bacteria and endotoxin translocation. We believe that possible reasons of intestinal bacteria and endotoxin translocation caused by THS are as follows. (1) Barrier dysfunction of body's intestinal immune: when THS occur, ischemia, hypoxia, and microcirculation cause damage of intestinal mucosal result in damage to the immune function so that invading bacteria and toxin cannot be effectively removed. (2) Ecological barrier dysfunction of body's intestinal tract: THS could bring about the body's intestinal microcirculation, blooms of Gram-negative bacteria, dysbacteria, and damage to the ecological barrier dysfunction of intestinal tract. (3) Dysfunction of body's intestinal mechanical barrier: THS can lead to ischemia, hypoxia, congestion, and microcirculation of intestinal mucosa, the formation of DIC, dysfunction of intestinal mechanical barrier, destruction of normal tight junctions between cells, and increased permeability of cells. All the above factors cause that bacteria and toxins enter the blood circulation through damaged intestinal mucosa, and that endotoxemia occurs.
TNF-α is a pro-inflammatory mediator. Endotoxin can promote the produce of a large amount of TNF-α, resulting in myocardial contractile function decline and myocardial damage. In this experiment, TNF-α blood levels in rats in the A2 group significantly increased, and myocardial damage was most severe. In contrast, TNF-α blood levels in rats in the A2 group significantly decreased, and myocardial damage was mitigated. These results indicate that when THS occurs, myocardial damage have some sort of link with TNF-α levels.
A study has shown that toxic effects of endotoxin are exerted mainly through TNF-α which serves as a "link". TNF-α plays a key role in myocardial injury of rats with sepsis [14]. TNF-α not only mediates a variety of biological toxicity of endotoxin, but also contribute to a cascade out of control, forming a complex network system, and causing inflammation that can lead to organ dysfunction [15].
Heart is not only an important target organ of TNF-α, but a place where TNF-α is produced. Studies have shown the following TNF-α toxicities: 1. TNF-α can lead to uncoupling and degradation of cardiac troponin, resulting in reduced contractility. 2. TNF-α can prompt myocardial cells to produce reactive oxygen species which destroy the cell membrane integrity. 3. TNF-α can promote myocardial apoptosis, causing ventricular remodeling and arrhythmia, and is involved in cardiac remodeling, resulting in heart failure. 4. TNF-α can promote the proliferation of fibroblasts, leading to decline in myocardial compliance. 5. TNF-α can induce lysosomal enzyme release [16], destroying myocardial electronic transfer system and the function of mitochondria, and leading to the production of new radicals [17]. An excessive amount of radicals can reduce the GSH-Px activity in the serum, and subsequently reduce the antioxidant capacity and radical scavenging capacity, promoting lipid peroxidation and damage of biomembranes [18]. 6. TNF-α can cause the formation of microthrombosis in myocardial vessels, microcirculation disorders and dysfunction [19], leading to myocardial necrosis. 7. TNF-α cam promote the production of chemokines and secondary inflammatory cytokines, which activate endothelial cells, and promote the expression of adhesion molecules. 8. TNF-α can induce the transient considerable rise in cardiac Ca2+ [20], causing myocardial hypertrophy [21], and ultimately bringing about decreased myocardial contractility, and heart dysfunction.
Antioxidant levels and the antioxidant enzyme system are vulnerable to multiple factors [22]. SOD is a kind of oxygen free radical scavenger, and MDA may reflect the degree of lipid peroxidation. SOD and MDA contents in myocardial tissues can reflect the extent of tissue damage. Although fluid resuscitation preformed after THS can improve myocardial blood supply, it aggravates myocardial ischemia-reperfusion injuries, and even leads to myocardial infarction. Cascades mediated by TNF-α and other molecules play an important role in myocardial ischemia-reperfusion injury.
In this experiment, TNF-α and CK-MB in the serum, and MDA contents in myocardial tissues in the A2 group significantly increased while SOD significantly reduced. Inflammatory cell infiltration appeared in cardiomyocytes, with thinner myocardial fibers and myocardial fiber necrosis. A study has found that TNF-α toxicities can be completely blocked by its monoclonal antibody [23]. In this experiment, compared with the A2 group, TNF-α levels in the A3 group significantly reduced (P<0.05) with reduced CK-MB in the serum, increased SOD contents, decreased MDA in myocardial tissues (P<0.05), and mitigated myocardial damages. These results indicate that TNF-α plays an important role in THS, and that its monoclonal antibody have a protective effect on myocardial tissues. The mechanism is possibly related to the antagonism of TNF-α toxicities.
Acknowledgements
This study is supported by Natural science funding program of Bengbu Medical College, Anhui Province (No. Byky1237).
References
- Allgower M. Traumatic hemorrhagic shock. Chirurg 1974; 45: 103-106.
- von Asmuth EJ, Maessen JG, Cj VDL. Tumour necrosis factor alpha (TNF-alpha) and interleukin 6 in a zymosan-induced shock model. Scand J Immunol 1990; 32: 313-319.
- Tang X, OReilly A, Asano M. p53 peptide prevents LITAF-induced TNF-alpha-mediated mouse lung lesions and endotoxic shock. Curr Mol Med 2011; 11: 439-452.
- Jin H, Yang R, Marsters SA. Protection against rat endotoxic shock by p55 tumor necrosis factor (TNF) receptor immunoadhesin: comparison with anti-tnf monoclonal antibody. J Infect Dis 1994; 170: 1323-1326.
- Conrad U, Plagmann I, Malchow S. Elpylated anti-human TNF therapeutic single-domain antibodies for prevention of lethal septic shock. Plant Biotechnol J 2011; 9: 22-31.
- Yuan G, Gong Z, Zhou X. Epigallocatechin-3-Gallate Ameliorates Alcohol-Induced Liver Injury in Rats. Int J Mol Sci 2006; 7: 204-219.
- Yahyavi H, Kaykhaii M, Hashemi M. A rapid spectrofluorimetric method for the determination of malondialdehyde in human plasma after its derivatization with thiobarbituric acid and vortex assisted liquid–liquid microextraction. Rsc Adv 2015; 6: 2361-2367.
- Li N, Huang HQ, Zhang GS. Effect of 5-AZn-2 -deoxycytidine on proliferation of human lung adenocarcinoma cell line A549 in vitro. Asian Pac J Trop Med 2013; 6: 982-985.
- James MF. Volume therapy in trauma and neurotrauma. Best Pract Res Clin Anaesthesiol 2014; 28: 285-296.
- Minamino T. Cardioprotection from ischemia/reperfusion injury: basic and translational research. Circ J 2012; 76: 1074-1082.
- Yao Y. Several serious problems in the pre-hospital emergency trauma which are worth discussion. J Traum Surg 2012; 14: 297-299.
- Wu Y, Gu EW, Zhu Y, Zhang L, Liu XQ. Sufentanil limits the myocardial infarct size by preservation of the phosphorylated connexin 43. Int Immunopharmacol 2012; 13: 341-346.
- Zhang L. Clinical studies on traumatic hemorrhagic shock. Guide Chin Med 2010; 8: 221-223.
- Zhang W, Wei W, Cao H. Expression and effects of tumor necrosis factor-a in septic myocardial injury in rats. Chin J Gerontol 2014; 34: 3682- 3684.
- Liu Z, Gao F. Regulatory effects of reduced glutathione on Inflammatory cytokines related with hemorrhagic shock acute lung injury and antioxidant factors and its protective effects on lung injury research. Pract J Cardiac Cerebr Pneum Vasc Dis 2013; 21: 38-40.
- Zhu W, Chen H, Lu Q. Protective effects of pentoxifylline on myocardium in rats with endotoxemia. J Emerg Med 2007; 16: 811-813.
- Yue M, Xu B, Li Y. Changes and significance of IL-12p70, TNF-a, IL-1b, IL-6, IL-8, and IL-10 in the plasma of sepsis patients. Chin J Traum 2012; 8: 1117-1119.
- Li D, Lv C, Wang Y. Experimental studies on effects of traumatic hemorrhagic shock on the bodys antioxidant levels. Pract J Med Pharm 2016; 33: 617-620.
- Ding L, Lu D, Shi X. TNF antagonists in the treatment of severe trichlorethylene induced dermatitis. Chin J Dermatol 2014; 47: 243-246.
- Wang GJ, Wang HX, Yao YS, Guo LY, Liu P. The role of Ca2+/calmodulin-dependent protein kinase II and calcineurin in TNF-Îα-induced myocardial hypertrophy. Braz J Med Biol Res 2012; 45: 1045-1051.
- Wang G, Yao Y, Wang H. Tumor necrosis factor -a induces myocardial hypertrophy in suckling rats via PI3K-IP3R-Ca2 + pathway. Chin J Pathophysiol 2016; 32: 21-26.
- Ma B, Gao Y, Pan G. Effects of luteolin on nitric oxide in the plasma and its antioxidant capacities in model rats with hyperlipemia. Chin J Gerontol 2014; 34: 2176-2178.
- Yang F, Yang X. Kinetic study of the interaction between tumor necrosis factors and soluble receptors. Anal Chem 2013; 41: 664-669.