- Biomedical Research (2011) Volume 22, Issue 3
The effects of melatonin on oxidative stress markers in an animal model of radiocontrast-induced nephropathy
Ayşegül Bayır1*, Hasan Kara2, Aysel Kıyıcı3, Halil Kıyıcı4, Ahmet Ak11Associate Professor, Department of Emergency Medicine, Selçuklu Faculty of Medicine, Selcuk University, Konya, Turkey
2Emergency Physician, Department of Emergency, Konya State Hospital, Konya, Turkey
3Assistant Professor, Department of Biochemistry, Selçuklu Faculty of Medicine, Selcuk University, Konya, Turkey
4Assistant Professor, Department of Pathology, Selçuklu Faculty of Medicine, Selcuk University, Konya, Turkey
- *Corresponding Author:
- Aysegul Bayir
Selcuk University, Selcuklu Faculty of Medicine
Department of Emergency Medicine
Selcuklu, Konya 42060 Turkey
Accepted May 01 2011
Abstract
We investigated the effects of melatonin on erythrocyte and renal tissue superoxide dismutase (SOD) and malondialdehyde (MDA) levels in rabbits after the administration of a radiocontrast agent. Twenty-four New Zealand rabbits were divided into four groups, six rabbits in each: control, sham, hydration, and hydration plus melatonin. Rabbits in the control group were sacrificed after the extraction of renal tissue. All other rabbits received a single dose of IV diatrizoat sodium (10 mL/kg). In the hydration group, saline (10 mL/kg IV) was infused at 6 hour intervals. In the hydration plus melatonin group, melatonin (10 mg/kg IV) and saline (10 ml/kg IV) were administered at 6 hour intervals. Venous blood samples were obtained from the rabbits before, and 48 and 72 hours after diatrizoat sodium administration to measure serum urea, creatinine, sodium, potassium, calcium, and erythrocyte MDA levels and SOD activities. Renal tissues were removed at the end of 72 hours, and tissue MDA levels and SOD activities were determined. At 72 hours, erythrocyte MDA concentrations of the hydration plus melatonin animals were significantly lower than those of the sham and hydration groups. However, erythrocyte and renal tissue SOD activities were significantly higher in the hydration plus melatonin group than the other groups. Renal tissue MDA levels of the hydration plus melatonin group were significantly lower than those of the sham and hydration groups. Melatonin has favorable effects on lipid peroxidation and antioxidant status in this animal model of radiocontrast nephropathy.
Keywords
radiocontrast-induced nephropathy, melatonin, oxidative stress, superoxide dismutase, malondialdehyde
Introduction
Impairment in renal function due to exposure to a radiocontrast substance is usually mild and transient. Contrast-induced nephropathy (CIN) is the third most common cause of new-onset acute renal failure in hospitalized patients. Although CIN occurs in 2% of the general population with normal renal function, its incidence in patients with prior renal dysfunction has been reported to be as high as 80% [1,2]. End-stage renal dysfunction necessitating dialysis occurs in 0.5-12% of CIN patients. CIN caused by iodine contrast agents is a serious complication leading to prolonged hospitalization, increased mortality, and increased healthcare costs [2,3].
Given these problems related to the use of radiocontrast agents, effective and cost-saving solutions for the prevention and treatment of CIN have been urgently sought. Depletion in renal blood flow, hypoxic damage and direct cellular toxicity are proposed pathophysio-logical mechanisms involved in the development of CIN. Reactive oxygen species are thought to play an important role, and thus antioxidants have been tested for their ability to prevent the development of CIN [4-6]. In this study, we compared the effects of hydration with and without melatonin, a potent antioxidant, on erythrocyte and renal tissue superoxide dismutase (SOD) activities and malondialdehyde (MDA) levels in rabbits after the administration of a radiocontrast agent.
Materials and Methods
Experimental Procedure
This laboratory-based controlled experimental animal study was performed after approval of the Experimental Animal Ethics Committee of our university. In our animal research laboratory, twenty-four New Zealand rabbits, weighing 3300-4500 g, were divided into four groups in a randomized fashion, six rabbits in each: control, sham, hydration, and hydration plus melatonin. Venous blood samples were obtained from all animals and then all were anesthetized with ketamine (50mg/kg IM) and xylazine HCl (15 mg/kg IM). These same doses of anestethics were used during laparatomy and at sacrifice at the end of the 72-hour study period. Renal tissue was extracted from the control group after laparotomy at the end of 72 hours. The sham group animals were given 10 ml/kg diatrizoate sodium IV. After radiocontrast administration, no other intervention was carried out on the sham group, i.e. CIN was induced without any post-radiocontrast protective measures being implemented. The hydration group animals were given 10 ml/kg of diatrizoate sodium IV, then normal saline (10 ml/kg IV) every six hours for 72 hours. The hydration plus melatonin group animals were given 10 ml/kg of diatrizoate sodium IV, then 10 mg/kg of melatonin dissolved in ethanol, in addition to normal saline (10 ml/kg IV) every six hours for 72 hours. Venous blood samples were obtained just prior to and 48 and 72 hours after radiocontrast administration.
Serum samples were tested for urea, creatinine, sodium, potassium and calcium levels, and whole blood samples were used for analysis of erythrocyte SOD activities and MDA levels. Seventy-two hours after administration of the radiocontrast agent, renal tissues were extracted and the animals were sacrificed.
Biochemical Analysis
Erythrocyte Lysates
After centrifugation of whole blood samples for 10 minutes at 3,000 rpm, plasma portions were aspirated. Then, the erythrocytes were washed four times with 0.9% NaCl solution: 3 ml of saline solution was used per mL of erythrocyte suspension and centrifugation was performed for 10 minutes at 3,000 rpm for each wash. The washed and centrifuged erythrocytes were made up to 2 mL in volume with cold redistilled water, then were stirred and left to stand at +4º C for 15 minutes. A 25-fold dilution of the lysate was prepared with 0.01M phosphate buffer (pH 7.0) so that the final dilution of the erythrocytes was 1:100.
Renal tissue samples were rinsed with ice cold saline and the blood and clot remnants were removed after the extaction. Subsequently, the samples were blotted on filter paper and stored at -80º C until biochemical analysis. Tissue samples were weighed and homogenized in 1.15% KCl (10% g/mL) on ice. These homogenates were used for MDA analysis. The remaining tissue samples were homogenized in Tris buffer (pH 7.5), and centrifuged at +4º C and 11,000 rpm for 20 minutes. Supernatants were used for SOD analysis. Protein content of the tissue samples was determined by the Bradford method [7]. SOD enzyme activities were given as U/mg protein.
MDA Analysis
MDA levels were determined by the method of Ohkawa [8] which depends on the reaction of lipid peroxidation products with thiobarbituric acid, resulting in metabolites with a maximum absorbance at 532 nm on spectrophoto-metry. Serial dilutions of 1,1,3,3 tetraethoxypropane were used to obtain a standard absorbance versus concentration curve and MDA concentrations of the tissue samples were determined from this curve. For tissue levels of lipid peroxidation products, nmol MDA/g wet tissue was used. Hemoglobin contents of the erythrocyte samples were determined by the cyanomethemoglobin method [9] and erythrocyte MDA concentrations were given as nmol/g Hb.
Superoxide Dismutase Activity
Superoxide dismutase enzyme activities were determined with RANSOD (Randox Laboratories, Antrim, UK) superoxide dismutase assay kits. This assay employs xanthine and xanthine oxidase (XOD) to generate super-oxide radicals which react with 2-(4-iodophenyl)-3-(4-nitrophenol)-5-phenyltetrazolium chloride (INT) to form a red formazan dye. Superoxide dismutase activity is then measured by the degree of inhibition of the reaction. One unit of SOD is that which causes a 50% inhibition in the rate of reduction of INT. SOD activities of tissue samples were given as U/mg protein and erythrocyte SOD activities were given as U/g Hb.
Histopathological Examination
3 μm thick sections were obtained from formalin fixed, paraffin-embedded tissue samples. Sections were stained with H&E and PAS. Pathological examination of these samples included comparison of groups according to presence of inflammatory reaction, tubular epithelial damage, brush border integrity and glomerular structure disturbance.
Statistical Evaluation
SPSS version 15.0 (SPSS, Inc., Chicago, USA) was used for statistical evaluation of the data. Kruskal-Wallis variance analysis and Mann Whitney U testing with Bonferroni correction were performed for comparison of the parameters. Results were given as mean±SD, and p values less than 0.05 were considered statistically signifi-cant.
Results
Renal Function Tests
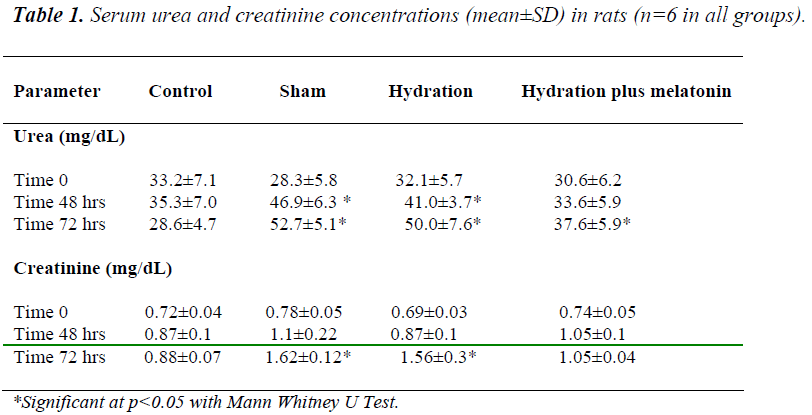
Baseline urea and creatinine concentrations were similar in all groups. We did not observe any significant differences between the groups regarding serum electro- lyte concentrations at any time point (time 0, 48 hours and 72 hours) during the experiment. At 48 hours, urea and creatinine concentrations were lower in the hydration plus melatonin group than in the sham and hydration groups (p=0.041 and 0.045 respectively), but similar to those of the control group. At 48 hours, urea and creatinine levels were higher in the hydration group than in the control group (p=0.026), but similar to those of the sham group. At the end of 72 hours, urea and creatinine levels in the sham and hydration groups were similar, but levels were significantly lower in the hydration plus melatonin group compared to the sham and hydration groups (p=0.02 and p=0.03, respecttively, Table 1).
Lipid Peroxidation Products
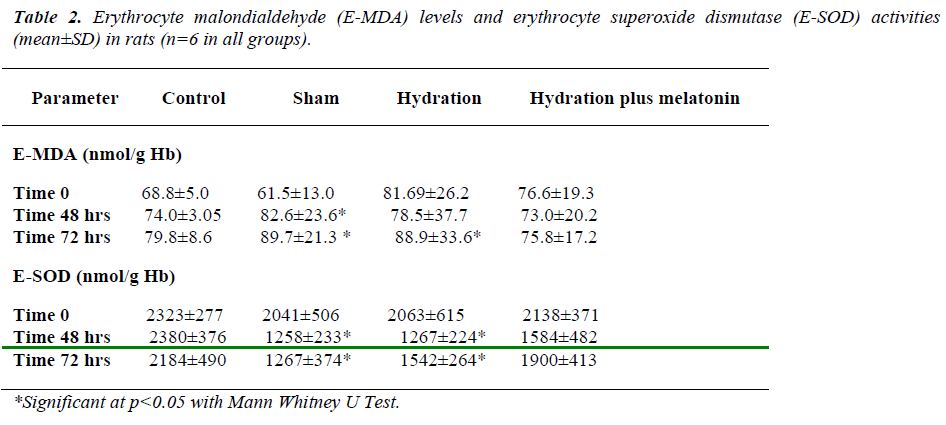
Baseline erythrocyte MDA levels and SOD activities were similar in all groups. At the end of 48 hours, erythrocyte MDA concentrations were as follows: the hydration plus melatonin group showed significantly lower MDA levels than the sham group (p=-0.021), but there was no significant difference between control, hydration, and hydration plus melatonin groups. Erythrocyte SOD activities were significantly lower in the sham group than the other three groups (p=0.012, p=0.03, p=0.02, respectively). Erythrocyte SOD activities of the control, hydration, and hydration plus melatonin groups were not significantly different from each other.
At 72 hours, erythrocyte MDA concentrations of the hydration plus melatonin group were significantly lower than those of the sham and hydration groups (p=0.015 and p=0.02). However, SOD activities in the hydration and sham groups were not significantly different from each other, but both groups had significantly higher MDA levels than the control group (p<0.05). Mean erythrocyte SOD activities of the hydration plus melatonin group were significantly higher than the control, sham, and hydration groups at the end of 72 hours (p=0.02, 0.017, and 0.02, respectively, Table 2).
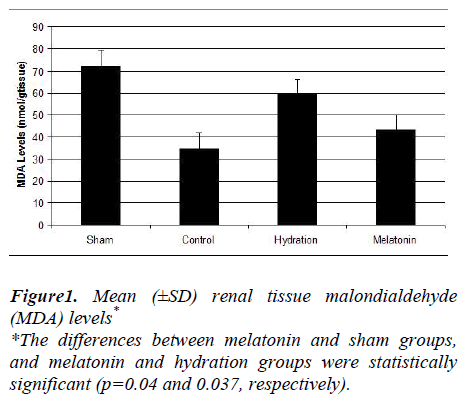
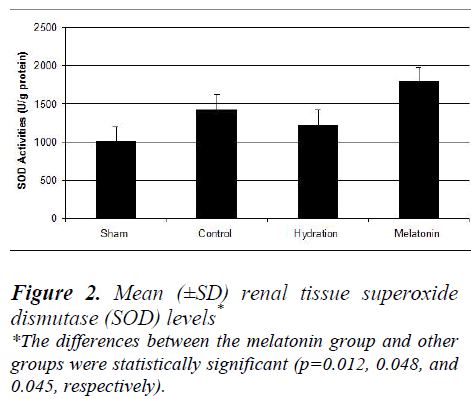
The hydration plus melatonin group had significantly lower levels of renal tissue MDA at 72 hours than the sham and hydration groups (p=0.04 and 0.037) but its levels were similar to those of control group (p=0.074). Renal tissue MDA levels were similar in the sham and hydration groups, but they were significantly higher in the hydration plus melatonin group compared to the control, sham and hydration groups (p=0.048, 0.012 and 0.045, respectively). However, the renal tissue SOD activities in the other three groups were not significantly different from each other (Figures 1 and 2).
Histopathological Examination
Light microscopic examination revealed no significant difference between the study and control groups; none of the samples had any significant inflammatory reaction nor glomerular structural disturbances. The luminal brush border was observed in all samples. Minimal tubular epithelial damage was seen in almost all samples, including the control samples (Figures 3, 4, 5, 6).
Discussion
Iodinated radiocontrast agents are toxic to renal cells; although hyperosmolar ionic radiocontrast agents like diatrizoate are more dangerous than hypoosmolar nonionic radiocontrast agents. The mechanisms of toxicity are unclear, but are thought to involve cellular energy insufficiency, disturbances in calcium homeostasis, defects in tubular polarity of the cells, and apoptosis. In-vitro studies investigating the cytotoxic effects of different radiocontrast agents on renal cells suggested that intercellular connections were damaged after diatrizoate application. A hypertonic extracellular environment causes fragmentation in DNA and oxidative stress due to production of reactive oxygen species [10]. Oxidative damage has been reported to participate in the progression and complications in renal disease and CIN. The red blood cells (RBCs) of uremic patients may have increased oxidative damage. The activities of different antioxidant enzymes and the levels of several antioxidants or lipid peroxidation products in RBCs are usually determined to estimate the oxidative stress in renal disease [11,12].
CIN results from direct renal tubular toxicity and renal medullary ischemia. Administration of contrast medium increases the production of nephrotoxic oxygen free radicals. Some antioxidants like N-acetylcysteine (NAC) and vitamin C inhibit ischemic cell death in the kidney [2,13]. Melatonin, which is secreted by pineal gland in verte-brates, was previously thought to be involved only in sleep and circadian rhythm regulation. Later, it was found to also be involved in a variety of physiological processes and to have anti-inflammatory, immunogenic, oncostatic and antifibrotic functions. Melatonin is also a powerful antioxidant and has protective effects against both reactive oxygen and nitrogen species. Its antioxidant effects are demonstrated mainly by its ability to decrease the formation of free radicals and increase of antioxidant enzymes. The antioxidant potential of melatonin is stro-nger than that of vitamin C, vitamin E, and glutathione [13-20].
After intravenous administration of a typical radiocontrast agent, hypoxic damage due to vasoconstriction and free radical formation increases serum creatinine levels steadily until peaking at 2-5 days. Major risk factors for CIN development are existing renal dysfunction and diabetes mellitus. Minor risk factors include congestive heart failure, dehydration, hypotension, hypoxia, age, smoking, hypercholesterolemia, concomitant use of nonsteroidal anti-inflammatory drugs, dose of radio-contrast agent, ionic and hyperosmolar characteristics of the agent, repeated doses at short intervals and usage of radiocontrast for abdominal imaging [21].
The ability of saline hydration to prevent CIN was investigated in a randomized controlled trial carried out on patients undergoing cardiac catheterization [22]. In patients who received IV hydration for 12 hours before exposure to radiocontrast agent, urea and creatinine levels 24 and 48 hours after catheterization were lower than patients who had normal oral water intake. They concluded that hydration with IV saline decreased the incidence of acute renal failure and renal dysfunction caused by radiocontrast agents.
In another study of chronic renal failure patients who underwent imaging with a hypoosmolar nonionic radiocontrast agent, the effects of this agent on serum creatinine levels were compared between oral and IV hydration groups. Theophylline or furosemide was added to the hydration protocols. Oral hydration was found as effective as IV hydration, but neither theophylline nor furosemide provided any additional benefit to IV hydration alone [23].
Among our four groups of animals, hydration with saline plus melatonin was the most effective strategy in preventing renal failure associated with diatrizoate, as measured by serum, whole blood, and tissue markers. Allaqaband, et al. compared the preventive effects of a 0.45% NaCl infusion with or without oral administration of fenoldopam and NAC on 123 patients to whom a radiocontrast agent was given for a cardiovascular procedure. Fenoldopam and NAC were given orally 4 hours before the intervention, in which a nonionic, hypoosmolar radiocontrast agent was used. Creatinine concentrations at the end of 24 and 48 hours were similar in the saline, and saline with fenoldopam and NAC groups [24]. In a parallel study, oral administration of NAC before the procedure showed results similar to those of hydration on prevention of CIN [25]. However, high dose IV NAC given before administration of a radiocontrast agent to patients with mild renal dysfunction preserved renal function [26].
Because adenosine is a key mediator in the development of CIN, researchers have tested adenosine antagonists like theophylline as agents to protect renal tissues from the damaging effects of radiocontrast agents. Results are conflicting; one study found that theophylline with hydra-tion decreased the incidence of CIN [27], but another found no advantage of theophylline over hydration alone [28].
Vitamin C was tested as a protective agent against the development of CIN in two studies [29,30]. In one, urea and creatinine levels were significantly decreased and creatinine clearances were increased in the vitamin C group compared to the control group [29], but in the other, no significant difference was found between patients who were hydrated and given either vitamin C or placebo [30]. Neither of these studies investigated the effects of vitamin C on free oxygen radical formation. In-vitro experiments with radiocontrast agents have found that antioxidants like 2-mercaptoethane sulphonate (MESNA) decreased tissue damage caused by free oxygen radicals on renal proximal tubules [6]. To date, research on antioxidant agents for the prevention of CIN has been limited to MESNA, NAC and vitamin C. Our current study is the first to investigate melatonin for this purpose.
In our study, erythrocyte MDA concentrations at the end of 72 hours were significantly lower and erythrocyte SOD concentrations were higher in the hydration plus melatonin group than the sham and hydration groups. This finding suggests that melatonin causes an increase in intracellular scavenger activity against lipid peroxidation. In the same fashion, but in renal tissue samples, MDA levels were significantly lower and SOD activities were higher in the hydration plus melatonin group than in the sham and hydration groups.
Histoptahological examination of our study revealed no significant differences between the control and study groups. Past studies found same results; for example, the protective role of erythropoietin administration against radiocontrast-induced nephropathy was evaluated in a study and it was reported due to light microscobic examination of the renal tissue sections that acute tubular injury was similar in all groups [31]. In another study, the effects of NAC on radiocontrast nephropathy were investigated and moderate to severe tubular lesions were observed in the group given radiocontrast [32]. But in that study, all of the rats were deprived of water for 24 hours prior to administration of the radiocontrast agent. Its histopathological findings may have been caused by the increased toxic effects of radiocontrast agent on renal tissue in the setting of dehydration. In a study of clonidine as a protective agent against radiocontrast-induced nephropathy in mice, the sham group had cortical proximal tubular necrosis, vacuolization and apoptosis, but the mice pretreated with clonidine had significantly less proximal tubular necrosis [23]. On histopathologic examination of the renal tissue samples, we did not observe any significant differences between the control and experimental groups. However, increased tissue levels of MDA were found in the sham group. The hydration plus melatonin group had significantly lower levels of renal tissue MDA at the end of 72 hours than the sham and hydration groups; in fact, its levels were similar to those of the control group. Mean renal tissue MDA levels were also similar in the sham and hydration groups. Renal tissue SOD activities were significantly higher in the hydration plus melatonin group compared to the control, sham and hydration groups. Thus, adverse effects due to the increase in oxidative stress caused by radio-contrast were mitigated by endogenous antioxidants such as SOD, resulting in protection of the renal tissues from acute tubular injury.
Tissue oxidative stress is a result of an imbalance in oxidant and antioxidant status. In our study, lower renal tissue lipid peroxidation levels in melatonin plus hydration group, compared to sham and hydration groups suggested that melatonin, either by its own antioxidant potential or by increasing the levels of antioxidant enzymes, provided this beneficial effect. This therapy should be tested for its effectiveness in preventing CIN in other animal models.
Limitations: The subject numbers of all groups was small. Results of the study may be change with large numbers of subjects. But ethical committee was not allowed for more subjects.
We can conclude that intravenous melatonin with hydration has favorable effects on lipid peroxidation and antioxidant status in radiocontrast-induced renal tissue damage more than hydration alone, in this animal model. Administration of melatonin with hydration is more beneficial than hydration alone in protecting erythrocytes and preserving renal function.
References
- Rihal CS, Textor SC, Grill DE et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002; 105 (19): 2259-2264.
- Brick R, Krzossok S, Markowetz F et al. Acetylcys- teine for prevention of contrast nephropathy: meta- analysis. Lancet. 2003; 362: 598-603.
- McCullough PA, Adam A, Becker CR et al. Epidemiol- ogy and prognostic implications of contrast-induced nephropathy. Am J Cardiol 2006; 98 Suppl 6A:5K-13K.
- McCullough PA. Radiocontrast-induced acute kidney injury. Nephron Physiol. 2008; 109:61-72.
- Persson PB, Hansell P, Liss P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. 2005; 68:14-22.
- Haeussler U, Riedel M, Keller F. Free reactive oxygen species and nephrotoxicity of contrast agents. Kidney Blood Pres Res. 2004; 27: 167-171.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95: 351-358.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utili- zing the principle of protein-dye binding. Anal Biochem. 1976; 72: 248-254.
- Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70: 158-169.
- Haller C, Hizoh I. The cytotoxicity of iodinated radiocontrast agents on renal cells in vitro. Invest Radiol. 2004; 39:149-154.
- Stoya G, Klemm A, Baumann E et al. Determination of autofluorescence of red blood cells (RbCs) in uremic patients as a marker of oxidative damage. Clin Nephrol. 2002; 58 (3):198-204
- Mohamed A, El-far Mohamed A, Bakr Sami E et al.Glutathione peroxidase activity in patients with renal disorders. Clin Exp Nephrol. 2005; 9:127–131.
- Solis HJA, Solis MP. Melatonin and oxidative stress.Rev Esp Enferm Dig 2009; 101:453-459.
- Tahan G, Akin H, Aydogan F et al. Melatonin ameliorates liver fibrosis induced by bile-duct ligation in rats. Can J Surg. 2010; 53: 313-318.
- Maestroni GJM. T-helper-2 lymphocytes as a periphe- ral target of melatonin. J Pineal Res. 1995; 18: 84-89.
- Shiu SYW, Li L, Xu JN et al. Melatonin induced inhibition of proliferation and G1:S cell cycle transitiondelay of human choriocarcinoma JAr cells: possible involvement of MT2 (MEL1B) receptor. J Pineal Res. 1999; 27: 183-192.
- Cuzzocrea S, Zingarelli B, Gilad E et al. Protective effect of melatonin in carrageenan induced models oflocal inflammation: Relationship to its inhibitory effecton nitric oxide production and its peroxynitrite scaven- ging activity. J Pineal Res. 1997: 23:106-116.
- Tan DX, Manchester LC, Reiter RJ, Plummer BF, Hardies LJ, Weintraub ST. et al. A novel melatonin metabolite, cyclic 3-hydroxymelatonin: a biomarker ofin vivo, hydroxyl radical generation. Biochem Biophys Res Commun. 1998; 253: 614-670.
- Stascia P, Ulanski P, Rosiak JM. Melatonin as a hydro- xyl radical scavenger. J Pineal Res. 1998; 25:65-66.
- Lee MC, Chung YT, Lee JH et al. Antioxidant effect of melatonin in human retinal neuron cultures. ExpNeurol. 2001; 172: 407-415.
- Lindholt JS. Radiocontrast induced nephropathy. Eur J Vasc Endovasc Surg. 2003; 25:296-304.
- Trivedi HS, Moore H, Nasr S et al. A randomized pros- pective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron ClinPract. 2003; 93: 29-34.
- Dussol B, Morange S, Laundoun A et al. A randomized trial of saline hydration to prevent contrast nephropathy in chronic renal failure patients. Nephron Dial Trans- plant. 2006; 21: 2120-2126.
- Allaqaband S, Tumuluri R, Malik AM et al. Prospec- tive randomized study of N-acetylcysteine, fenoldo- pam, and saline for prevention of radiocontrast-inducednephropathy. Catheter Cardiovasc Interv. 2002; 57: 279-283.
- Boccalandro F, Amhad M, Smalling RW et al. Oral acetylcysteine does not protect renal function from moderate to high doses of intravenous radiographic contrast. Catheter Cardiovasc Interv. 2003; 58:336-41.
- Baker CS, Wragg A, Kumar S et al. A rapid protocol for the prevention of contrast-induced renal dysfunc-tion: the RAPPID study. J Am Coll Cardiol. 2003; 41: 2114-2118.
- Huber W, Ilgmann K, Page M et al. Effects of theophyline on contrast material-induced nephropathyin patients with chronic renal insuffiency: controlled, randomized, double-blinded study. Radiology. 2002; 223: 772-779.
- Erley C, Duda SH, Rehfuss D et al. Prevention of radiocontrast-media-induced nephropathy in patients with preexisting renal insufficiency by hydration incombination with the adenosine antagonist theophyll- ine. Nephrol Dial Tranplant. 1999; 14: 1146-1149.
- Spargias K, Alexopoulos E, Kyrzopoulos S et al. Asco- rbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation. 2004; 110:2837-2842.
- Boscheri A, Weinbrenner C, Botzek B et al. Failure of ascorbic acid to prevent contrast-media induced nephropathy in patients with renal dysfunction. Clin Nephrol. 2007; 68: 279-286.
- Goldfarb M, Rosenberger C, Ahuva S et al. A role for erythropoietin attenuation of radiocontrast induced acute renal failure in rats. Ren Fail. 2006; 28:345-350.
- Yenicerioglu Y, Yilmaz O, Sarioglu S et al Effects of N-acetylcysteine on radiocontrast nephropathy in rats. Scand J Urol Nephrol. 2006; 40: 63-69.
- Billings FT 4th, Chen SW, Kim M et al. Alpha2-Adre-nergic agonists protect against radiocontrast-induced nephropathy in mice. Am J Phy Renal Phy. 2008; 295: F741-8.