Research Article - Biomedical Research (2017) Volume 28, Issue 18
The effects of HIF-1α and VEGF on wound healing in diabetic mice
Chujia Lin*, Guoshu Yin, Miaoqiong Ou and Shaoyu Zheng
Department of Endocrinology and Metabolism, the First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong, PR China
- *Corresponding Author:
- Chujia Lin
Department of Endocrinology and Metabolism
The First Affiliated Hospital of Shantou University Medical College
Shantou, Guangdong, PR China
Accepted date: August 29, 2017
Abstract
Objective: To investigate the effects of Hypoxia Inducible Factor-1 alpha (HIF-1α) and Vascular Endothelial Growth Factor (VEGF) on wound healing of diabetic mice.
Methods: Streptozotocin (STZ) was used to induce establishing wound healing model group of type 1 diabetic mouse in the experiment. The protein expression of HIF-1α and VEGF at different time point were measured in the wound healing model group of type-I diabetic mouse and normal control group by use of Western blotting and then relative mRNA expression quantity of HIF-1α and VEGF were determined with relative quantitative method.
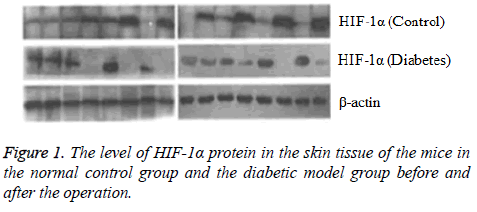
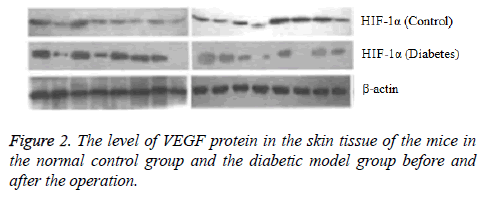
Results: Before the operation, the difference between the diabetic model group and the normal control group in HIF-1α and VEGF expression as well as the relative expression quantity of mRNA was not statistically significant (P>0.05). At the fifth day of finishing operation, compared with the preoperative level the protein expression levels of HIF-1α and VEGF increased in the normal control group (P<0.05) and decreased in the diabetic model group (P<0.05) and the relative expression quantity of HIF-1α and VEGF mRNA in the normal control group was obviously higher that in the diabetic model group (P<0.05).
Conclusion: HIF-1α and VEGF play important roles in diabetic wound healing and the decrease of their expression level may lead to lack of angiogenesis, which will delay the healing of diabetic wound.
Keywords
Hypoxia inducible factor-1 alpha (HIF-1α), Vascular endothelial growth factor (VEGF), Diabetes mellitus, Mice, Wound healing
Introduction
Diabetic wounds are easy to become protracted course of disease with repeated-onset in clinical trials. It will develop into refractory skin diseases and ulcers and the patients may be faced with the risk of amputation [1]. How to effectively treat the diabetic wound is one of the biggest challenges in current clinical practices. Relevant data showed that an important pathological mechanism of difficult diabetic wound healing is the disorder or delay in local angiogenesis of the wound [2]. HIF-1α is an important nuclear transcription factor and plays a very important role in wound healing through their active participation in a variety of pathological and physiological process of energy metabolism and angiogenesis [3,4]. On the other hand, Vascular Endothelial Growth Factor (VEGF) can induce angiogenesis and promote revascularization in vivo [5]. At present, there are few reports about the expression as well as role of HIF-1α and VEGF in the repair of diabetic skin wound. The study was aimed to investigate the expression of HIF-1α and VEGF at different time points of mice in the diabetic model group and normal control group and to explore its role in the mechanism of diabetic wound healing.
Materials and Methods
The study objects were a total of 20 male Kunming mice weighed about 25-30 g and provided by Laboratory Animal Center of our Hospital. Streptozotocin (STZ) (Beijing Solarbio Technology Co., Ltd.) blood glucose meter (Beijing Yicheng Limited by Share Ltd.), gel imager (Beijing Saizhi Startup Science and Technology Co., Ltd.) total RNA Extraction Reagent (Guangzhou Nucrel Biotechnology Co., Ltd.), ultraviolet spectrophotometer (Beijing Chang Department of Instrument Automation Technology Co., Ltd.), Real-Time Fluorescent Quantitative PCR instrument (Xi’an Tianlong Science and Technology Co., Ltd.), mouse anti-human HIF-1α polyclonal antibody (alpha Shanghai Rong Wei of Industrial Co., Ltd.), mouse anti human VEGF polyclonal antibody (Jiangsu Champrosay Biotechnology Co., Ltd.), mouse anti human β-actin polyclonal antibody (Beijing Huaxia Ocean Technology Co., Ltd.), total protein extracting kit (Shanghai Chunshi Biological Technology Co., Ltd.). PCR primers of HIF-1, VEGF and beta-actin were obtained from Shanghai Biotechnology Co., Ltd.
Methods
The study objects were divided into diabetic model group and normal control group with 15 mice in each group. Establishment of the diabetic model group: an intra-peritoneal injection of 45 mg/kg STZ was performed in 10 mice for constant 5 days, at the fifth day of finishing injection, the mice were in the fasted 10 h, cannot help but water. Tail vein blood of mice was taken followed by the measurement of fasting blood glucose with a blood glucose meter. If the level of fasting blood sugar was greater than 11.1 mmol/L, the mice with polydipsia, polyphagia and polyuria were included in the diabetic model group. Two-months after the model establishment, intra-peritoneal anaesthesia were performed in the mice to cut the skin tissue of size 1 cm × 1 cm on the back.
Establishment of the normal control group: An intraperitoneal injection of 45 mg/kg saline was performed in 10 mice for constant 5 d, at the fifth day of finishing injection, the mice were in the fasted 10 h, cannot help but water. Two months after establishing the control model, intraperitoneal anesthesia was performed in the mice to cut the skin tissue of size 1 cm × 1 cm on the back.
Wound tissues of 5 mice were collected from each group at the time of respectively before operation and at the fifth day of wound treatment. The protein expression of HIF-1α and VEGF at different time point was measured in the two groups by use of Western blotting and the relative mRNA expression quantity of HIF-1α and VEGF relative quantitative method. The determination of HIF-1 and VEGF protein expression by Western blotting. The total protein in the skin tissue of mice was extracted with conventional method. Proteins and standard proteins of each group were collected and transferred to nitrocellulose film after SDS-PAGE electrophoresis with a blocking time of 1 h in sealing fluid at 37°C followed by the addition of the mouse anti-human HIF-1α polyclonal antibody (concentration 1:1000), mouse anti human VEGF polyclonal antibody (concentration 1:1000) and sheep anti rabbit IgG second antibody (concentration 1:15000). The nitrocellulose membrane was mixed with the Super signal substrate for 2 min and then exposed to the X-ray film in the darkroom after color development followed by the scanning and observation with a gel imager. Measurement of relative expression quantity of HIF-1 and VEGF mRNA by qRT-PCR relative quantitative method skin tissues of the experimental mice were collected in which the total RNA was fragmented according to the instructions of TRI pure total RNA extraction reagent and the RNA level was measured by UV spectrophotometer for the determination of its purity. After the synthesis of cDNA by reverse transcription kit, specific primer PCR amplification was performed with the methods provided by Lifeng’s team [6]. Data collection was performed by quantitative PCR software, and the relative expression quantity of the target gene was calculated according to the following formula: ΔCt=Ct target gene-Ct β-actin, the higher the ΔCt value, the lower the relative expression quantity of the target gene.
Statistical analysis
Data were statistically analyzed on SPSS 20 and assessed by ttest, P<0.05 suggested there was difference of statistical significance.
Results
Establishment of diabetic wound model mice
After the eighth week of induction by STZ, the fasting blood glucose level was 17.04 ± 0.52 mmol/L in the experimental mice, consistent with the requirements of diabetic mice. Each mouse had the characteristics of diabetes such as more food, polydipsia and polyuria with no death. In this study, the vital signs of each mouse were normal and no mice died after the performance of wound formation in the diabetic model mice and the normal control mice. Comparison of HIF-1 and VEGF proteins expression determined by Western blotting. Before the operation, there was no significant difference in the HIF-1 and VEGF proteins expression between the normal control group and the diabetic model group (P>0.05). At the fifth day after operation, compared with the preoperative level the protein expression levels of HIF-1α and VEGF increased in the normal control group (P<0.05) and decreased in the diabetic model group (P<0.05) (Figures 1 and 2).
Comparison of relative expression quantity of HIF-1α and VEGF mRNA
At the fifth day of finishing operation, the relative expression quantity of HIF-1α and VEGF mRNA in the normal control group was obviously higher that in the diabetic model group (P<0.05) with no statistically significant difference between the two groups before the operation as shown in Table 1.
| Group | n | HIF-1α | Intragroup | p | VEGF | Intragroup | p | ||
|---|---|---|---|---|---|---|---|---|---|
| Before operation | 5 d after operation | Before operation | 5 d after operation | ||||||
| Control group | 5 | 5.15 ± 0.32 | 6.41 ± 0.57 | 7.002 | <0.05 | 4.98 ± 0.41 | 5.67 ± 0.55 | 5.603 | <0.05 |
| Diabetic model group | 5 | 5.08 ± 0.25 | 4.66 ± 0.36 | 6.382 | <0.05 | 4.87 ± 0.55 | 4.12 ± 0.64 | 5.994 | <0.05 |
| Intragroup | 0.213 | 7.657 | 0.195 | 6.348 | |||||
| p | >0.05 | <0.05 | >0.05 | <0.05 | |||||
Table 1. Expression of HIF-1α and VEGF mRNA in mice with skin wound.
Discussion
The incidence of increased tends to gradually increase in recent years and the patients once diagnosed with diabetes, are prone to suffer from skin damage resists closure, which then evolves into chronic refractory wounds followed by serious consequences. How to effectively treat diabetic wound is becoming more and easier for clinicians to pay attention to. At present, the treatment of diabetic wound includes infection control, local medicine and surgical treatment, but the therapies of special efficiency have yet to be found [7-9]. Relevant data show that the difficulty in diabetic wound treatment is in that the lack of blood supply in local tissue of the wound leads to the obstruction of angiogenesis and makes it difficult to produce new granulation tissue [10]. Studies have shown that HIF-1α plays an important role in angiogenesis [11]. In the condition of enough oxygen, HIF-1α will be degraded after hydroxylation of proline hydroxylase; on the contrary, it will be easy for HIF-1α to accumulate in the cell without degradation, enter the nucleus via a nuclear localization signal, activate the downstream gene expression and regulate a variety of factor like angiogenesis in vivo. Research has indicated that with the occurrence of skin wounds the level of HIF-1α has a higher level of expression in vivo and then up regulate the expression level of angiogenic factors like VEGF, which can induce migration and proliferation of vascular endothelial cells, increase blood vessel permeability as well as angiogenesis and improve local blood supply of skin wounds [12-14].
The results of this study show that in diabetic wound healing both the expression level of VEGF and HIF-1α of skin tissue and the relative expression quantity of HIF-1α and VEGF in the normal control group was significantly higher than those in the diabetic model group (P<0.05). It may be because high level of glucose is much likely to facilitate the increase of superoxide and induces the accumulation of acetone in cells, thus reducing transcriptional activity of HIF-1α. Some studies have suggested that the main factors contributing to diabetic foot are decreased expression of HIF-1α and VEGF, lower vascular density, insufficient blood supply and impaired wound healing [15].
To sum up, HIF-1α and VEGF play a vital role in the healing of diabetic wound and the decrease of their expression level may lead to the lack of angiogenesis and delay the healing course. This study has certain guiding significance in treatment of diabetic wound healing and in the future we can further explore whether transgenic technology can increase the expression of HIF-1 and VEGF and then promote the healing of diabetic wound as soon as possible.
References
- Nishikori Y, Shiota N, Okunishi H. The role of mast cells in cutaneous wound healing in streptozotocin-induced diabetic mice. Archiv Dermatol Res 2014; 306: 823-835.
- De MT, Conget P, Becerra-Bayona S. The role of bone marrow mesenchymal stromal cell derivatives in skin wound healing in diabetic mice. Plos One 2017; 12: e0177533.
- Jacobi J, Jang JJ, Sundram U. Nicotine accelerates angiogenesis and wound healing in genetically diabetic mice. Am J Pathol 2002; 161: 97-104.
- Li X, Li D. Study on the effect of ointment on diabetic mice skin ulcer and its mechanism. Guangming J Chin Med 2014; 29: 46-48.
- Hayashi D, Kawakami K, Ito K. Low-energy extracorporeal shock wave therapy enhances skin wound healing in diabetic mice: a critical role of endothelial nitric oxide synthase. Wound Repair Regeneration 2012; 20: 887-895.
- Jing L, Li S, Li Q. Expression and significance of hypoxia inducible factor -1 signaling system in diabetic wound healing. Guangdong Med J 2014; 35: 1154-1156.
- Xie P, Xue X, Zhang Ji B. Effects of nerve growth factor combination with Insulin on wound healing with II degree deep scald and the expression of HIF-1α and VEGF in diabetic rats. J Chin Phys 2011; 13: 33-37.
- Jung KH, Yi TG, Mi KS. Therapeutic effect of human clonal bone marrow-derived mesenchymal stem cells in severe acute pancreatitis. Archiv Pharm Res 2015; 38: 742-751.
- Zeng Y, Jian H, Gao B. Effect of external application of insulin on expression of HIF-1 and VEGF in deep partial thickness burn wound of diabetic rats. J Chongqing Med Univ 2010; 35: 860-864.
- Rošic R, Kristl J, Baumgartner S. Protein loaded hydrogels as dressing for moist wound healing. Farmacevtski Vestnik 2010; 61: 187-193.
- Liu L, Marti GP, Wei X. Age-dependent impairment of HIF-1 alpha expression in diabetic mice: Correction with electroporation. J Cell Physiol 2010; 217: 319-327.
- Li J, Si J, Liu H. Effects of astragaloside IV on the maturation of myocardial neovascularization and the expression of HIF-1 and VEGF in infarcted mice. Chin Heart J 2017; 29: 269-275.
- Chen L, Gajendrareddy PK, Dipietro LA. Differential expression of HIF-1 alpha in skin and mucosal wounds. J Dental Res 2012; 871-876.
- Cao J. Effects of MEBO on the expression of HIF-1 and VEGF in wound healing tissue. J Guiyang College Trad Chin Med 2013; 34: 47-48.
- Ling S, Birnbaum Y, Nanhwan MK. MicroRNA-dependent cross-talk between VEGF and HIF1α in the diabetic retina. Cell Signalling 2013; 25: 2840-2847.