Research Paper - Asian Journal of Biomedical and Pharmaceutical Sciences (2016) Volume 6, Issue 59
The Effect of Radiopacifier in Portland Cement for Repairing Furcal Perforations
Jiang Ying1,3, Zhiqiang Zhou1,3, Yan Wang2,3, Xiaohui Xu2,3 and Juhong Lin1,3*
1Chongqing Key Laboratory for Oral Diseases and Biomedical Sciences, P.R China
2Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, P.R China
3The Affiliated Stomatology Hospital, Chongqing Medical University, Chongqing, 401147, P.R. China
- *Corresponding Author:
- Lin J
Chongqing Key Laboratory for Oral Diseases and Biomedical Sciences P .R. China
Tel: +86- 23 -88860090
E-mail: cqjuhonglin@sina.com
Accepted October 13, 2016
Abstract
This study was designed to evaluater adiopacity, interface micrographs, and leakage mediated by zirconium oxide (ZO) placed into white Portland cement (PC) as a furcal perforation sealing material. Perforations were created in the floors of pulp chambers in 56 freshly human maxillary and mandibular molars and randomly divided into four experimental groups, each containing 12 teeth, and a negative control group containing 8 teeth. The experimental groups were prepared using 20% ZO plus PC, PC, ProRoot mineral trioxide aggregate (MTA), and 20% bismuth oxideplus PC. First, radiopacity was assessed through X-ray. Second, the concentration of leaked glucose was measured at day 1, 2, 4, 7, 10, 15, and 21 using a glucose oxidase method. Third, using scanning electron microscopy (SEM), we showed the interface between each material and teeth. The data were evaluated using the Kruskal-Wallis test. The results suggest that three materials showed sufficient radiopacity, the only exception is the lower radiopacity of PC, and ZO could be considered as an alternative radiopacifier to be added in PC, without changing the ability of sealing the furcal perforations.
Keywords
Furcation perforation repair, Sealingability, Radiopacity, Zirconium Oxide, Portland cement.
Introduction
Furcal perforation may be the result from pathological conditions (e.g., tooth in absorption or decay) or iatrogenic factors (e.g., improperpulp cavity preparations). The prognosis of a treatment depends on the perforation site, the size of damages, adjacent periodontal conditions, and sealing materials. An ideal sealing material must exhibit favorable sealing ability, high biocompatibility, nontoxicity to the pulp tissue, antibacterial properties, anticipated stability in tissue fluids, adequate mechanical strength, short-time setting, and favorable handling properties [1,2].
Clinical investigations have demonstrated that mineral trioxide aggregate (MTA) is a good material for the treatment of furcal perforations. Moreover, it has been extensively applied in dentistry as reparative material for root-end filling, pulp-capping, pulpotomy, and traumatized teeth within complete apexification [3-6]. MTA may be an ideal material that has showed excellent physicaland biological properties such as biocompatibility, high pH, radiopacity, reasonable sealing ability, and the capacity to promote pulpand periradicular tissue repair. For instance, it consistently inducers generation of the periodontal ligaments, the apposition of a cementum-like material, and the formation of bone. However, MTA has a long setting time, which makes it difficult to handle. The high cost also restricts its clinical application [5,6].
Recently, it has been confirmed that PC plusbismuth oxide to make the mix radiopaque [7]. This has generated many scientific interests in comparing MTA with PC and in the evaluation of PC as a low-cost alternative to MTA. Several studies have demonstrated some similarities between both materials in terms of their biocompatibility, composition, mechanism of action, and cytotoxicity [8-11]. The only exception is the lack of radiopacity in PC. The lower radiopacity in PC is a major concern. When it is considered as a substitute for MTA [5-7,12]. Radiopacity is one of the most important properties to be observed during some dental procedures such as root canal therapies. An ideal root canal repair material should present sufficient radiopacity to distinguish filling materials from surrounding anatomic structures [1]. Thus, the radiopacity of PC associated with the various radiopacifying agents was evaluated. Among the tested radiopacifiers, bismuthoxide showed the highest radiopacity, which made bismuth oxide a widely used material for many MTA-type dental filling materials [7,12,13]. However, it has been demonstrated that dental discoloration can be caused by white MTA induced by bismuth oxide in teeth previously contacted with water or sodium hypochlorite solution [14]. Furthermore, bismuth oxide, as a radiopacifying agent in MTA, interferes with MTA properties by increasing its porosity, consequently lowering its resistance [15,16]. Formosa et al. reported that bismuth oxide drastically increased the setting time of the test cements in phosphate-containing solutions [17].
So, the association of PC with other radiopacifier agents has also been studied [15-21]. Antonijevic et al. have demonstrated that PC with the addition of at least 10% bismuth oxide and20% ZO or ytterbium trifluoride displayed greater radiopacity value than the recommended 3 mm aluminum [15]. Filler loading of 30% ZO to PC mixed at a water to cement ratio of 0.3 resulted in a material with comparable properties to MTA [19]. Chen et al. have shown that the radiopacifier bismuth oxide/ yttria-stabilize dzirconia (85/15) had higher radiopacity, but similar cell viability, than purebismuth oxide [20]. Several studies have showed that the negative properties of bismuth oxide [14-18]. PC associated with ZO was shown to have excellent physicochemical, biocompatibility and mechanical properties [18-20,22,23]. However, it is not clear whether ZO would be the best radiopacifying agent associated with PC in the treatment of furcal perforations. Therefore, in this study, we evaluated the radiopacity, interface micrographs and the leakage by ZO placed into white PC as a furcal perforation sealing material.
Materials and Methods
Selection of teeth
This study was approved by the Ethics Committee, School of Stomatology, Chongqing Medical University, China. Fifty six freshly human maxillary and mandibular molars were selected from the Stomatological Hospital of Chongqing Medical University. After removing soft tissue tags and calculus, the teeth were autoclaved and stored in normal saline solution (containing 0.2% sodium azide) no longer than 14 days. The teeth were selected based on their well-developed morphologies such as intact furcation, complete root formation, and the absence of fracture lines. To maximize specimen standardization, we used the teeth that have 2.2 to 2.5 mm dentin thickness at the furcation. We excluded teeth with root caries, fused root, and damaged pulp chamber floors.
Teeth preparation
The teeth were decoronated 3 mm above the cement enamel junction. Standard access cavities were prepared. Pulp tissue and debris were removed. The pulp cavity was irrigated with sterile saline and dried with oil-free air. The canal orifices were located. Artificial perforations were created in the center of the pulp chamber floor by using a size 3 round diamond bur (carbide burs FG-3, SS White Burs, Lakewood, NJ) in a high-speed hand piece with water spray. The teeth were then allowed to dry at room temperature. Sticky wax was used to seal the orifice of each canal. Two coats of nail polish were applied over the entire tooth surface, especially in the furcal location. A silicone impression material (Dentsply, Addlestone, UK) was mixed to provide a matrix that simulates the bony socket and the perforations. Teeth were placed into the unset silicone and removed when polymerization occurred.
Repair of the perforations
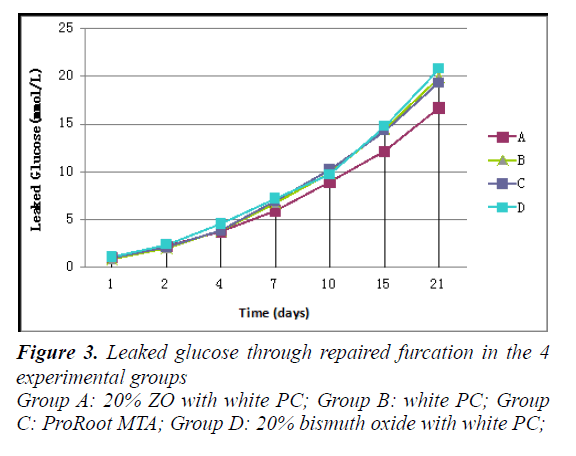
The teeth were randomly divided into four experimental groups containing 12 teeth and one negative control group containing 8 teeth. The groups were designated as: Group A: 20% ZO (Sigma Aldrich, StLouis, MO, USA) with white PC; Group B: white PC (Aerbo Cement Corporation, Anqing, China); Group C: ProRoot MTA (Dentsply, Tulsa, USA); Group D: 20% bismuth oxide (Sigma Aldrich, St. Louis, MO, USA) with white PC; and Group E: negative control (only preparing the access cavities without creating furcal location).
In groups A, B, C, and D, 1 g of each material was mixed with 0.35 mL of distilled water according to manufacturer’s recommendations. The mixed materials were placed into the perforation sites by using an Endogun (Dentsply, Tulsa, USA) and compacted with the Schilder Pluggers (Dentsply, Tulsa, USA). A cotton ball with saline solution was placed in the pulp chamber against the placed materials. In group E, standard access cavities were prepared in each tooth with a number 5 round bur (carbid burs FG-5, SS White Burs, Lakewood, NJ) in the high-speed hand piece to ensure that the furcation remains intact, All the samples were stored at 37%°C in 100% humidity for 72 h to allow the cement to set.
Assessment of the radiopacity
All samples for each tested material were used for an X-ray-based radiopacity assessment. Images were taken with a conventional X-raydevice rr (PLD5000B, Pulang, China) using 60 kVP, 10 mA, and an exposure time of 0.3 seconds. A distance of 20 mm was standardized as the distance from the cylinder to the periapical film in all samples (Kodak serie E -P21, China).
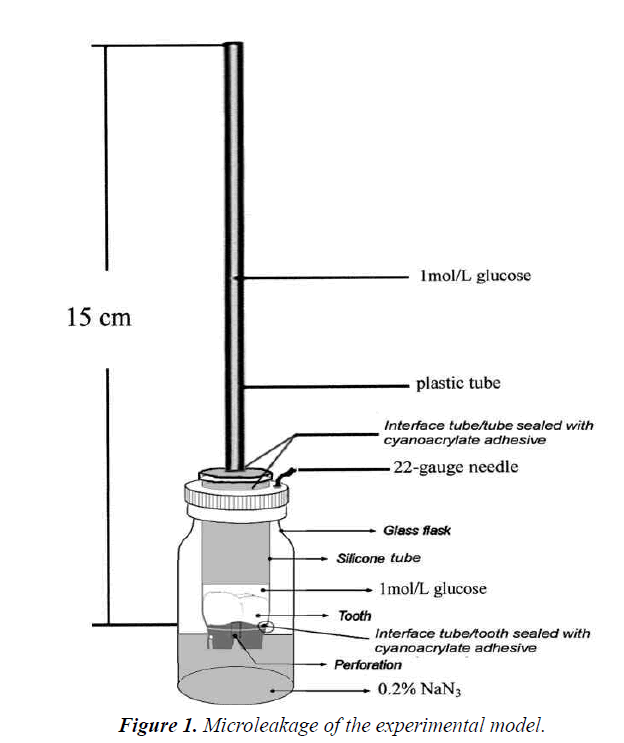
Measurement of microleakage
A quantitative method using glucose solution was chosen to assess the leakage, which was detailed in the previous studies [24,25]. As shown in Figure 1, the model was transferred to an incubator at 37°C in 100% humidity for the observation. At day 1, 2, 4, 7, 10, 15 and 21, a 10 μL sample solution was taken out from each glass bottle. Corresponding amount of distilled water was added into every tube to maintain the volume of 2 mL, either before or after drawing the sample. The samples were analyzed with an Automatic Biochemistry Analyzer (Modular P800, Roche, USA).
Scanning electron microscopy investigation
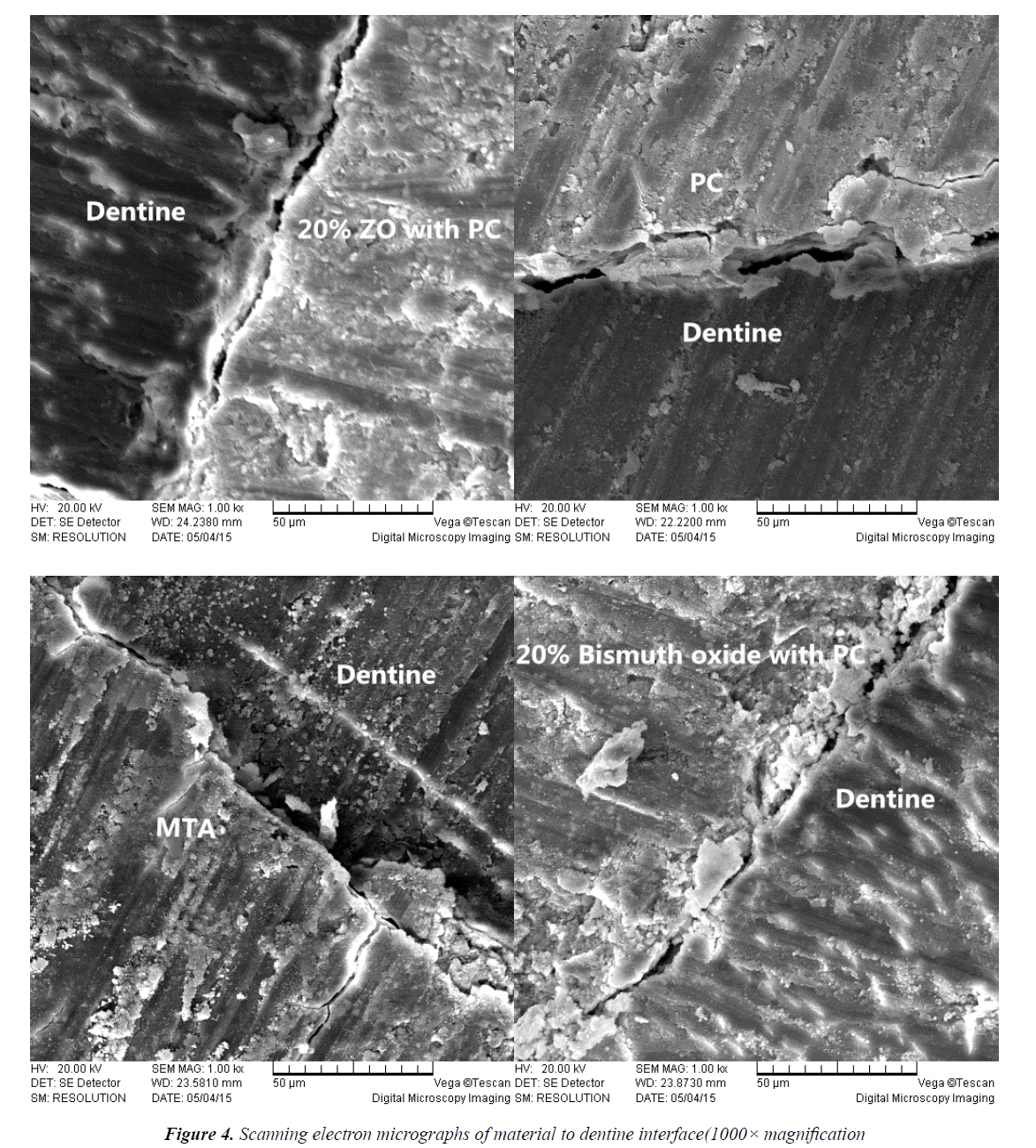
After leakage detection, the specimens were removed from the model. Using a hard tissue cutting machine (E300CP/400CS, EXAKT Vertriebs GmH, Germany), the materials were longitudinally cut to tooth interface. The dried longitudinal sections were coated with gold to a thickness of 20 nm using a Bio-Rad Sc5000 sputter coater (Fisons Instruments, Uckfield, UK). Mounted specimens were examined under a scanning electron microscope (QUANTA400, FEI, USA), which was maintained at approximately 20 Kv and 10-6 Torr under a high vacuum condition. Scanning electron micrographs of the material to tooth interface were captured at 1000-fold magnification.
Statistical analysis
Owing to the data having the nature of non-normal distribution, all values were analyzed by the Kruskal- Wallis test. The level of significance was set at a P value less than 0.05.
Results
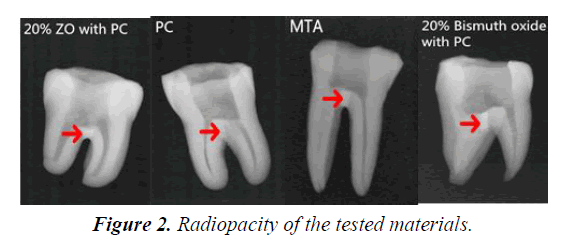
Biomaterials with sufficient radiopacity
As illustrated in Figure 2, MTA, 20% ZO with PC, and 20% bismuth oxide with PC present sufficient radiopacity to distinguish materials from surrounding anatomic structures on radiographs. However, PC showed lower radiopacity, indistinguishable from the dentine structures. The radiopacity of PC has been shown to be 0.86-2.02 mm aluminum [7,12], lower than the 3 mm aluminum recommended by the International Standards for Dental Root Canal Sealing Materials (ISO 6876 Section 7.8: 2007) [2].
Microleakage analysis
Figure 3 illustrates experimental findings of leakage. No glucose leakage was found in the negative control group from the beginning to the end of the experiments, indicating the reliability of our model. Leaked glucose was observed in each experimental group with an increasing tendency as time went on. The concentration of leaked glucose in the group of Group A (20% ZO+PC) was lower than other groups. Group D (20% Bismuth oxide+PC) seems to have the highest concentration. However, there was no significant difference among the experimental groups on each time points (P>0.05). Our results showed that the addition of ZO to PC did not affect the capacity to seal furcal perforations. Statistical analysis of the present study also showed that both PC and MTA demonstrateda similar ability (to seal furcal perforations) to that showed by De- Deus et al. in apolymicrobial leakage model and Gustavo et al. using a fluid filtration model [21,26].
Micrograph analysis
SEM was used in this study because it is an efficient method to examine surface topography [27]. The scanning electron micrographs of the material to tooth interface demonstrated changes in microstructural characteristics of the materials in contact with dentine. Figure 4 showed microscopic spaces and uneven clearance between the restoration and the dentine in each experiment group, which may lead to microleakage. The reasons for this may be the marginal gaps found frequently in perforation fillings, which reduce their sealing ability and create the conditions for bacterial and fluidmicro leakage from crown to apex.
Discussion
The addition of various radiopacifying agents was reported [15-20,22-23]. Bismuth oxide is a radiopacifier presented in MTA. However, several studies have shown that the association of bismuth oxide changed the color, microstructures, porosity, and solubility of PC [14-16].
For instance, Marina et al. presented dental discoloration caused by bismuth oxide in MTA in the presence of sodium hypochlorite [14]. Other materials have been evaluated as alternatives to bismuth oxide [15-20,22,23]. ZO provides proper radiopacity and cell viability. Also, ZO associated with PC presents radiopacity, compressive strength, reasonable setting times, water absorption, and solubility similar to ProRoot MTA [9,19]. Cutajar et al. Described PC replaced with 30% ZO mixed at a water/cement ratio of 0.3 resulted in an optimal combination [19]. In this study, we used 20% ZO with PC as the experiment material. There was 20% replacement of white Portland cement with micron-sized ZO particles reported to provider adiopacity values between 3.41 and 6.58 mm aluminum, which were conformed to International Standards [18]. We showed that the addition of ZO to PC promotes proper radiopacity, which also exceeds the minimum ISO standard of 3 mm aluminum for root canal sealers.
In previous studies, various methods have been developed to investigate leakage, such as dye leakage, fluid filtration, bacterial penetration, and protein leakage, most of which have both advantages and inherent draw backs [5,21,26,28]. In this study, we chose glucose solution as the leakage tracer and enzymatic glucose oxidase as the assay. The model quantifies the microleakage with high sensitivity because the molecular size of glucose is small. If glucose could enter the canal from the oral cavity, bacteria that might survive root canal preparation and obturation could multiply and potentially lead to periapical inflammation. The oxidation of glucose would be affected by several factors, such as the concentration of the glucose solution, the volume and the solubility of the materials.
Shahriar et al. reported that PC had better sealing ability than MTA, which should be recommended for repair of furcal perforation [28]. Several reports also indicated that MTA had better marginal adaptation than Glass Ionomer Cement, Intermediate Restorative Material, Super Ethoxybenzoic Acid and Amalgam [27,29-31]. On the contrary, another investigation demonstrated that Intermediate Restorative Material and Super Ethoxybenzoic Acid have had better marginal adaptation than MTA [32]. However, a recent study compared the marginal adaptation among White MTA, Gray MTA, and PC and showed no significant difference between the tested materials, regardless of improved marginal adaptation [33].
In summary, our study showed that the sealing ability promoted by all 4 materials was similar. ZO could be considered as an alternative radiopacifier to be added in PC, without changing the sealing ability at the furcal perforations. However, further research is needed to evaluate the behaviors of the radiopacifying agents associated with PC with respect to other physicochemical and biological properties and clinical applications of this type of materials.
References
- Walton RE, Torabinejad M. Principles and Practice of Endodontics,3rd edn.Oxford, UK: W B Saunders Co 2002.
- Baksi BG, SenBH, EyubogluTF. Differences in aluminum equivalent values of endodontic sealers: conventional versus digital radiography. J Endod. 2008; 34: 1101-1104.
- Material.International Standard; ISO 6876, 2nd ed. Geneva: ISO; 2007.
- Pace R, Giuliani V, Pagavino G. Mineral trioxide aggregate as repair material for furcal perforation: case series. J Endod. 2008; 34: 1130-1133.
- Al-Daafas A, Al-Nazhan S. Histological evaluation of contaminated furcal perforation in dogs' teeth repaired by MTA with or without internal matrix. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2007;103:e92-99.
- Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review--part II: leakage and biocompatibility investigations. J Endod. 2010;36:190-202.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400-413.
- Coutinho-Filho T, De-Deus G, Klein L, Manera G, Peixoto C.Radiopacity and histological assessment of Portland cement plus bismuth oxide. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2008; 106: e69-77.
- Shahi S, Rahimi S, Yavari HR, Mokhtari H, Roshangar L. Effect of mineral trioxide aggregates and Portland cements on inflammatory cells. J Endod. 2010; 36: 899-903.
- Viapiana R, Flumignan DL, Guerreiro-TanomaruJM, Camilleri J, Tanomaru-Filho M. Physicochemical and mechanical properties of zirconium oxide and niobium oxide modified Portland cement-based experimental endodontic sealers. IntEndod J. 2014;47:437-448.
- Ha WN, Bentz DP, Kahler B, Walsh LJ. D90: The Strongest Contributor to Setting Time in Mineral Trioxide Aggregate and Portland Cement. J Endod. 2015; 41: 1146-1150.
- Ahmed HM, Luddin N, KannanTP, Mokhtar KI, Ahmad A. Cell attachment properties of Portland cement-based endodontic materials: biological and methodological considerations. J Endod. 2014;40:1517-1523.
- Hungaro Duarte MA, de Oliveira EKadre GD, Vivan RR, GuerreiroTanomaruJM, TanomaruFilho M, de MoraesIG. Radiopacity of portland cement associated with different radiopacifying agents. J Endod. 2009;35:737-740.
- Mestieri LB, Tanomaru-Filho M, Gomes-Cornélio AL, Salles LP, Bernardi MI. Radiopacity and cytotoxicity of Portland cement associated with niobium oxide micro and nanoparticles. J Appl Oral Sci. 2014; 22: 554-559.
- Marciano MA, Duarte MA, Camilleri J. Dental discoloration caused by bismuth oxide in MTA in the presence of sodium hypochlorite. Clin Oral Investig. 2015; 19: 2201-2209.
- Antonijevic D, Medigovic I, Zrilic M, Jokic B, Vukovic Z, Todorovic L. The influence of different radiopacifying agents on the radiopacity, compressive strength, setting time, and porosity of Portland cement. Clin Oral Investig. 2014;18:1597-1604.
- Coomaraswamy KS, Lumley PJ, Hofmann MP. Effect of bismuth oxide radioopacifier content on the material properties of an endodontic Portland cement-based (MTA-like) system. J Endod. 2007;33:295-298.
- Formosa LM, Mallia B, Camilleri J. The effect of curing conditions on the physical properties of tricalcium silicate cement for use as a dental biomaterial. IntEndod J. 2012; 45: 326-336.
- GuerreiroTanomaruJM, Storto I, Da Silva GF, Bosso R, Costa BC, Bernardi MI, Tanomaru-Filho M. Radiopacity, pH and antimicrobial activity of Portland cement associated with micro- and nanoparticles of zirconium oxide and niobium oxide. Dent Mater J. 2014;33:466-470.
- Cutajar A, Mallia B, Abela S, Camilleri J. Replacement of radiopacifier in mineral trioxide aggregate; characterization and determination of physical properties. Dent Mater. 2011; 27: 879-891.
- Chen C, Hsieh SC, Teng NC, Kao CK, Lee SY. Radiopacity and cytotoxicity of Portland cement containing zirconia doped bismuth oxide radiopacifiers. J Endod. 2014; 40: 251-254.
- De-Deus G, Petruccelli V, Gurgel-Filho E, Coutinho-Filho T. MTA versus Portland cement as repair material for furcal perforations: a laboratory study using a polymicrobial leakage model. IntEndod J. 2006;39:293-298.
- Viapiana R, Guerreiro-TanomaruJM, Hungaro-Duarte MA, Tanomaru-Filho M, Camilleri J. Chemical characterization and bioactivity of epoxy resin and Portland cement-based sealers with niobium and zirconium oxide radiopacifiers. Dent Mater. 2014;30:1005-1020.
- Keskin C, DemiryurekEO, Ozyurek T. Color stabilities of calcium silicate-based materials in contact with different irrigation solutions. J Endod. 2015; 41: 409-411.
- Xu Q, Fan MW, Fan B, Cheung GS, Hu HL. A new quantitative method using glucose for analysis of endodontic leakage. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2005; 99: 107-111.
- Zou L, Liu J, Yin SH, Tan J, Wang FM. Effect of placement of calcium sulphate when used for the repair of furcation perforations on the seal produced by a resin-based material. IntEndod J. 2007; 40: 100-105.
- De-Deus G, Reis C, Brandão C, Fidel S, Fidel RA.The ability of Portland cement, MTA, and MTA Bio to prevent through-and-through fluid movement in repaired furcal perforations. J Endod. 2007; 33: 1374-1377.
- Xavier CB, Weismann R, de Oliveira MG, Demarco FF, Pozza DH.Root-end filling materials: apical microleakage and marginal adaptation. J Endod. 2005; 31: 539-542.
- Shahi S, Rahimi S, Hasan M, Shiezadeh V, Abdolrahimi M. Sealing ability of mineral trioxide aggregate and Portland cement for furcal perforation repair: a protein leakage study. J Oral Sci. 2009;51:601-606.
- Gondim E, Zaia AA, Gomes BP, Ferraz CC, Teixeira FB. Investigation of the marginal adaptation of root-end filling materials in root-end cavities prepared with ultrasonic tips. IntEndod J. 2003; 36:491-499.
- Shipper G, Grossman ES, Botha AJ, Cleaton-Jones PE. Marginal adaptation of mineral trioxide aggregate (MTA) compared with amalgam as a root-end filling material: a low-vacuum (LV) versus high-vacuum (HV) SEM study. IntEndod J. 2004;37:325-336.
- Torabinejad M, Smith PW, Kettering JD, Pitt Ford TR. Comparative investigation of marginal adaptation of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod. 1995;22:295-299.
- Tobon-Arroyave SI, Restrepo-Perez MM, Arismendi-EchavarriaJA, Velasquez-Restrepo Z, Marin-Botero ML, Garcia-Dorado EC. Ex vivo microscopic assessment of factors affecting the quality of apical seal created by root-end fillings. IntEndod J. 2007;40:590-602.
- Bidar M, Moradi S, Jafarzadeh H, Bidad S. Comparative SEM study of the marginal adaptation of white and grey MTA and Portland cement. AustEndod J. 2007; 33: 2-6.