Research Article - Environmental Risk Assessment and Remediation (2018) Volume 2, Issue 2
The effect of operational parameters on biosorption of Cd2+, Ni2+ and Cr6+ using Glycine max pod (Soya Bean).
Kabir A Sanusi1*, Nathaniel S Sunday1, Mohammed S Hassan1, Tahir A Abdulqadir2
1Department of Chemical Sciences, Faculty of Science, Federal University Kashere, Gombe State, Nigeria
2Department of Biological Science, Faculty of Science, Federal University Kashere, Gombe State, Nigeria
- *Corresponding Author:
- Kabir A Sanusi
Department of Chemical Sciences
Faculty of Science
Federal University Kashere
Gombe State
Nigeria
E-mail: adebayokabir@ymail.com
Accepted date: February 28, 2018
Abstract
The potential of glycine max pod (GMP) in removal of Cd2+, Ni2+ and Cr6+ ions from aqueous solutions was examined in a batch adsorption process with respect to several experimental conditions including pH of solution, contact time, GMP dosage, initial metal ions concentration, and temperature, etc. The characterization of GMP was performed by using FTIR and SEM techniques. The maximum uptake of Cd2+ (1.445 mg/g), Ni2+ (1.585 mg/g) and Cr6+ (1.594 mg/g) was observed when used 2.0g of GMP biomass, 25 mgL-1 of initial Cd2+, 50 mg/l of initial Ni2+ and 50 mg/l of initial Cr+6 concentration at pH 5, 6 and 3 and contact time of 90, 60 and 90 min at room temperature for Cd2+, Ni2+ and Cr6+ respectively. The experimental data were analyzed by the Langmuir, Freundlich, Temkin and Dubinin-Radushkevich (D-R) isotherm models. The monolayer adsorption capacity Of GMP was found to be 21.56 mg/g, 53.76 mg/g and 18.87 mg/g for Cd2+, Ni2+ and Cr6+ ions, respectively. The kinetics of the adsorption was tested using pseudo-?rst-order, pseudo-second-order, Elovich and intra-particle diffusion models. The results showed that the adsorption of Cd2+, Ni2+ and Cr6+ ions onto GMP proceeds according to the pseudo-second-order model.
Keywords
Glycine max, biosorption, cadmium, nickel, chromium, kinetics.
Introduction
Industrial wastewater contains high levels of heavy metals, and their potential health implications to man and his environment has been a subject of concern in environmental studies. Heavy metal ions discharged with industrial effluents usually find their way into receiving water bodies such as rivers, lakes and streams therefore increasing the amounts of these pollutants in such water bodies. This can have serious health implications on man ranging from cancer, hormonal imbalance to brain impairment. Therefore, industrial wastewater treatment for the removal of pollutants such as heavy metal ions, remains a challenge to both developed and developing countries of the world [1].
Cadmium ion (Cd2+), a non-essential, non-beneficial element to plants and animals is non-biodegradable and travels through the food chain. The major sources of Cd2+ released into the environment are effluents from electroplating, smelting and alloy manufacturing processes [2-4]. Cd2+ may be ingested through the food chain, through polluted soils in which food crops are grown and inhaled through smoking. The kidneys are the critical target organ after ingestion (renal dysfunction, hypertension and anemia). Cd2+ combines with sulfhydryl group in protein and restrains the activity of enzymes [2]. The permissible limits of Cd2+, Ni2+ is 0.05 mg/L [5].
Nickel ions (Ni2+) are non-biodegradable toxic heavy metals present in the effluents of silver refineries, electroplating, zinc base casting and storage battery industries. Acute poisoning of Ni2+ causes headache, dizziness, nausea and vomiting, chest pain, dry cough, respiratory problems and cyanosis [4]. The Ni2+ level in wastewater from mine drainage and metal finishing has been reported up to 130 mg/L [6,7].
Chromium (Cr6+) is a very toxic pollutant introduced into natural waters from a variety of effluents from dyeing and metal finishing industries [8]. Chromium occurs most frequently as Cr6+ or Cr3+ in aqueous solutions. Both valences of chromium are potentially harmful but hexavalent chromium poses a greater risk due to its carcinogenic properties [9]. High exposure of Cr6+ causes cancer in the digestive tract and lungs leading to cause gastric pain, nausea, vomiting, severe diarrhea, and hemorrhage [8]. Because its bioaccumulation in aquatic organisms, feeding on such aquatic organisms can lead to metal poisoning in man. According to the United Nation Environment Programme [10] the maximum permissible limits in wastewater and potable water are 1.0 mg/L and 0.05 mg/L for chromium (VI) respectively [11].
In waste water treatment, various technologies are available such as chemical precipitation, ion exchange, electrochemical precipitation, solvent extraction, membrane separation, coagulation, reverse osmosis, emulsion and adsorption are used. However, cost implication, huge sludge generation and environmental friendliness form the major techno-economic limitation of these techniques for water treatment. Therefore the development and improvement of absorption capacities of agricultural waste material for removal of toxic pollutants especially heavy metals is needed to reduce cost of purifying industrial wastewaters before disposal [12].
This research work is aimed at probing into the metal uptake ability of Glycine max pod (GMP) to ascertain potential of Glycine max pod (GMP) as a biosorbent in sequestering heavy metals from aqueous solution. For its objective, this study investigates the influence of some operating parameter such as the effect of pH, contact time, initial metal ion concentration and sorbent dosage on the uptake of Cd2+, Ni2+ and Cr6+ by Glycine max pod. The kinetics and equilibrium modeling of the system were also investigated.
Materials and Methods
Preparation of the biosorbent
The Glycine max pods (GMP) were collected at different location within a farmland in Agbonle village, Saki-East LGA of Oyo State. The GMP sample was washed to removed dirt and debris, air dried. The dried sample was grounded in a mechanical grinder to form a powder. The powder was sieved using a 0.45 mm mesh screen and stored in air tight plastic bottle to protect it from moisture before use in all the experiments.
Characterization of the biosorbent
The Specific Surface Area (SSA) of the sample was determined using the methods of Sears [13] and Iler [14]. The bulk density and specific gravity of the GMP sample was determined as described by Olu-Owolabi et al. [15]. The pH at the point zero charge (pH PZC) was determined as described by Unuabonah et al. [16]. This is important because it marks the pH where the surface functional groups do not contribute to the pH of the solution, and in an applied electric field, the electrophoretic mobility of the particles would be zero [17].
The Fourier Transform Infrared (FTIR) spectra of the GMP biosorbent were obtained using Perkin Elmer Spectrum1 FTIR spectrophotometer. About 0.1 g of the processed dry GMP biosorbent in powder form along with KBr were ground into fine particles and compressed into pellets was used to obtain the FTIR spectrum in the scanning frequency from 4100 to 400 cm-1.
Biosorption experiments
The synthetic metal ion (Cd2+, Ni2+ and Cr6+) solutions were prepared from AnalaR grade of their salts (CdCl2, Ni(NO3)2.6H2O and K2Cr2O7). A stock solution of 1000 mg/L was prepared by dissolving 1.5985 g, 4.9547 g and 2.8289 of Cd2+, Ni2+ and Cr6+ salts respectively in 200 cm3 distilled water and then made up to mark in a 1L standard volumetric flask. Working solutions of various concentrations were prepared from this stock solution as required. The pH of sorbate solutions were adjusted using 0.1 M HCl or 0.1M NaOH. All experiments were carried out in duplicates to enhance the integrity of analytical results. Blank experiments were conducted to estimate and correct for adsorptions to the walls of the apparatus used.
Batch experiments were carried out to determine metal sorption capacity Glycine max pod (GMP) at room temperature (28 ± 2)°C by suspending 2 g of GMP in separate 20 mL of 250 mg/L metal ion solution in a 60 mL polythene bottles. After the sorbent-sorbate mixtures were shaken on a mechanical shaker, biomass suspensions were filtered using whatman No.1 filter paper and concentration of metals in the clear supernatant filtrate solutions were analyzed using the Atomic Absorption Spectrometer (Buck Scientific 205) with air-acetylene flame on absorbance mode d equipped with hollow cathode lamps used for the metals.
After biosorption experiment, the metal ion concentratios adsorbed by GMP biomass in mg/g were calculated by using the following equation:
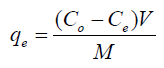 (1)
(1)
Where; Ce and Ce are the initial and equilibrium sorbate concentrations in solutions; qe, V and M are the amount of sorbate adsorbed (mg/g), volume of the solution (mL) used for incubation and mass (g) of biosorbent respectively.
Adsorption isotherms
Adsorption isotherms of Cd2+, Ni2+ and Cr6+ unto GMP biomass were obtained by batch experiments working at optimized adsorbent dose, pH and contact time as established after optimization of working parameters. Initial metal concentration was varied from 5 to 50 mg/L for all the metals. The amount of metal adsorbed q was determined using Equation 1. To obtain insights into the surface properties and degree of affinity of GMP for the metal ions and hence adsorption process, adsorption data were tested against the Langmuir, Freundlich and Dubinin- Redushkevich (D-R) equilibrium isotherms using the linear forms of these models in Eqs. (2), (3) and (4).
Langmuir: 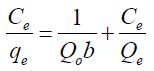 (2)
(2)
Freundlich: 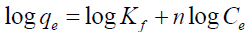 (3)
(3)
where; 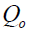 is the maximum adsorption capacity per unit weight of sorbent, b is a solute-surface interaction energy-related parameter, while
is the maximum adsorption capacity per unit weight of sorbent, b is a solute-surface interaction energy-related parameter, while 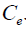 and
and 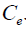 are the amount of solute adsorbed per unit mass of the adsorbent (mg/g) and equilibrium metal concentrations in the solution (mg/L); where
are the amount of solute adsorbed per unit mass of the adsorbent (mg/g) and equilibrium metal concentrations in the solution (mg/L); where 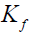 and n are the Freundlich model capacity factor and the isotherm linearity parameter, respectively.
and n are the Freundlich model capacity factor and the isotherm linearity parameter, respectively.
Dubinin-Radushkevich (D-R) isotherm [18]: This is a more general isotherm than the Langmuir and Freundlich and does not assume a homogeneous surface or constant adsorption potential. The linear form of the model is represented as follows:
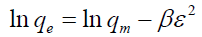 (4)
(4)
Where β is a constant related to the mean free energy of adsorption per mole of adsorbate (kJ2/mol2), capacity (mg/g).
Kinetic models
In describing the mechanism of the biosorption of Cd2+, Ni2+ and Cr6+ ions by GMP biomass and the potential rate determining steps, such as mass transport and chemical reactions, reactionbased and diffusion-based kinetic models were used to stimulate the experimental adsorption data, the pseudo-first-order, pseudosecond- order, Elovich, and Intra-particle diffusion models have been applied to the biosorption data. The Langergren pseudo first-order and pseudo second-order rate equations [19] were tested using their simplified equations in (4) and (5) respectively:
1. The pseudo first order kinetic model [19]:
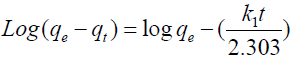 (5)
(5)
Where qe and qt are the amounts of metals sorbed (mg/g) at equilibrium and at time t, respectively; and k1 is the rate constant of pseudo-first-order sorption (min-1). The qe and rate constants k1 were calculated from the slope and intercept of the plot of log (qe- qt) vs. t.
2. The pseudo second order kinetic model as proposed by Ho and McKay [20]:
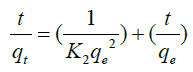 (6)
(6)
Where k2 is the rate constant of pseudo-second-order sorption. The pseudo-second-order rate constants k2 and qe values were calculated from the slope and intercept of the plots t/q vs. t.
3. Elovich model as described by Basha and Murthy as adopted by Olu-Owolabi et al. [21]:
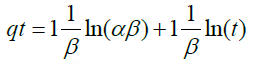 (7)
(7)
Where α is regarded to as the initial adsorption rate (mg/g min) and β is related to the extent of surface coverage and activation energy for chemisorption (g/mg). (α and β are constants).
4. The linearized form of the intra-particle diffusion model equation [22] was used as shown in Eq. (7):
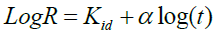 (8)
(8)
Where R is percentage metal ion adsorbed, t is contact time, α is gradient of linear plots and depicts the adsorption mechanism, Kid is intra-particle diffusion rate constant (h-1).
Results and Discussion
Characterization of biosorbent
Some of the physicochemical characteristics of the Glycine max pod (GMP) adsorbent used in this study were determined using methods described by Unuabonah et al. [16]. The value of the PZC of GMP biomass was measured to be at pH 6.81. The PZC value obtained above for GMP adsorbent indicates that it could be used to efficiently adsorb metal ions at pH values as low as 6.81 as the adsorbent becomes more negative at values above the PZC. The GMP biosorbent gave Cation Exchange Capacity (CEC) of 36.51 meq/100 g).
When viewed under a Scanning Electron Microscope (SEM), GMP biosorbent gave particles with regular structures and showed some white particles indicative of porosity of the surface of the biomass (Figure 1b).The SSA of GMP has been determined as 27.280 m2/g, while the bulk density and specific gravity are 0.575 g/cm3 and 0.176 g/cm3 respectively. The determination of GMP surface properties is necessary because the particle sizes are most likely heterogeneous and these properties aid in estimating the degree of metal adsorption.
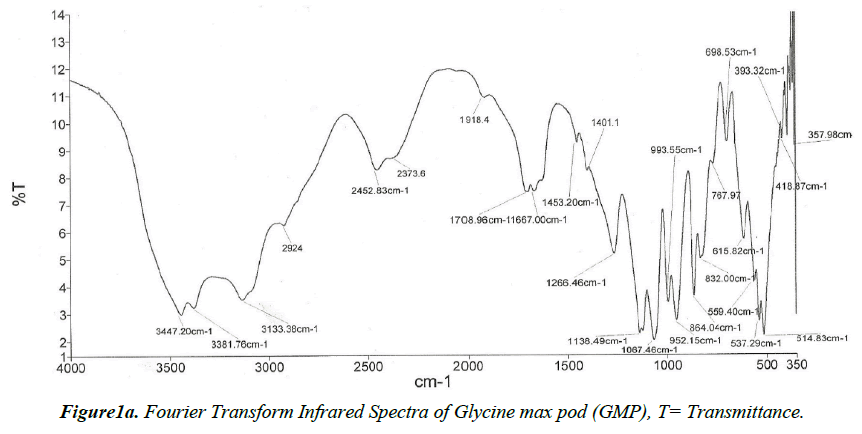
The FTIR (Fourier Transform Infrared Spectrograph) of the GMP biomass is shown in Figure 1a. Peaks appearing in the raw biomass were assigned various functional groups and bands in accordance with their respective wave numbers (cm-1). The sharp vibration peak at 3447 cm-1 was assigned to the stretching of O-H group due to inter- and intra-molecular bonding of polymeric compounds such as alcohols, phenols and carboxylic acids, as in pectin, cellulose and lignin. The broad range for this O-H band indicates the presence of ‘free’ hydroxyl groups of carboxylic acids [23]. The band 2924cm-1 is assigned the asymmetric C-H vibration in -COOH group of aliphatic acid [21,24]. The peak in the region of 2452 and 1266 cm-1 corresponding to the stretching vibrations of C-C or C=O or C-H or C-O in nonionic carboxyl groups such in esters and carboxylic acids is present in the GMP spectra. The peaks in the 1061 cm-1 is due to the presence of the C-C stretching polysaccharides [25]. Upon loading of the GMP sample with Cd2+, Ni2+ and Cr+6 ions, the 1708 cm-1 bands indicating the presence of a non-ionic carboxyl group appeared in the spectra. The appearance of the 1453 cm-1 and 1401 cm-1 band in the metal GMP-FTIR spectra may be attributed to a chemical interaction between the metals and the biomass. The shifts in the ionic carboxylate and hydroxylate anions observed after loading of the biomass may be attributed to changes in counter ions associated with these ions suggesting their contribution to metal uptake. Moreso, the structure of GMP biosorbent and presence of the outer –OH peak which suggests the possibility for the adsorption of Cd2+, Ni2+ and Cr+6 onto this site on this adsorbent [23] .
Effect of pH of solution
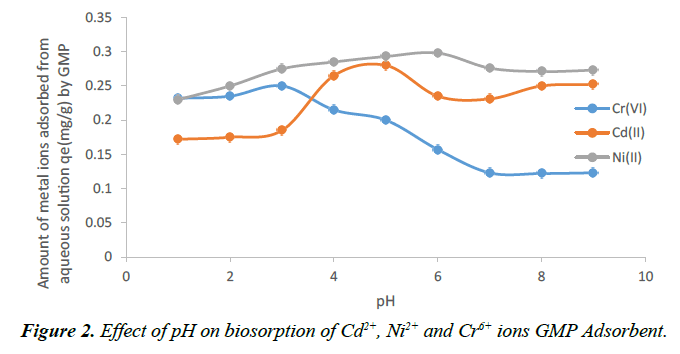
For biosorption, the pH of the aqueous solution is an important controlling parameter in the metal uptake process. Metal adsorption on surface of biomass materials has been described in terms of molecular mechanisms which may include cation exchange in the interlayer, and specific adsorption resulting from surface complexation. Hydrogen ions affect metal complexation because they have great affinity for adsorption sites [21]. Hence, pH value of a solution strongly influences not only the site dissociation of the biomass surface, but also the solution chemistry of the heavy metals. For this reason, the effect of pH on the uptake of Cd2+ ,Ni2+ and Cr+6 ions onto GMP was studied by varying pH values in range of 1 to 9 and the results were presented in Figure 2 respectively. It is clear that Cd2+ ion was effectively adsorbed in the pH range 4 and 5 with the highest amount adsorbed at pH 5. While for Ni2+ ion there was continuous increase in the amount metal ions adsorbed from pH 2 to 6 with the highest removal at pH 6. Similar trends were reported by Jimoh et al. [26-29]. In these cases, pH 5 was reported as the optimum pH for Cd2+ adsorption. This result therefore shows weak acidic pH favours Cd2+ biosorption onto GMP. The increase in the amount of metal ion adsorbed due to increase in pH may be explained on the basis of decrease in the competition between proton (H+) and the positively charged metal ion at the surface sites and by decrease in positive charge near the surface resulting in a lower repulsion of the adsorbing metal ion. This means that at higher H+ concentration, the sorbent surface becomes more positively charged thus reducing the attraction between the sorbent and metal cations [30]. In contrast, as pH increases, more negatively charged surface becomes available thus facilitating greater metal uptake and therefore metal sorption tends to increase significantly by increasing pH. Thus, Cd2+ and Ni2+ at pH values 5 and 6 have 98.7% and 96.0% removal respectively by GMP biosorbent.
However, for Cr6+ the highest amount of the metal ion adsorbed was at slightly strong acidic pH 3 which decreases significantly as solution pH increases. Similar trend was reported by Qaiser [31] This unusual adsorption at such low pH was explained to be as a result of the presence of HCrO4 as the dominant form of Cr+6 which was attracted by positively charged sites [32]. The further justification for higher removal of chromium at low pH is the reduction of Cr6+ to Cr3+ which was then sorbed by the biosorbent [32]. As a result of this reduction reaction, H+ was consumed and an increase in pH was observed at the end of the experiments. Thus, Cr+6 at pH 3 have 79.8% removal by the GMP biosorbent.
Effect of adsorbent dosage
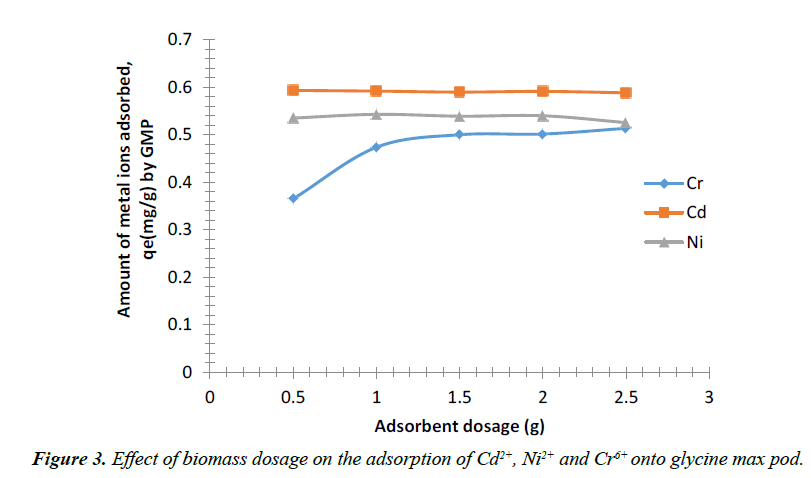
Figure 3 shows the effect of adsorbent dose on the percentage removal of Cd2+, Ni2+ and Cr+6 respectively. The percentage of Cd2+, Ni2+ and Cr+6 ions adsorbed by GMP increased with an increase in the weight of adsorbent from 0.5 to 3g of GMP after which the percentage adsorbed remained constant.
From the analysis of the experimental data obtained for metal ions, it was observed that the adsorption efficiency increased progressively with increase in adsorbent dose for Cr6+ ion up to a mass of 2 g after which the amount of metal adsorbed remain almost constant while for Ni2+, it was observed that the adsorption efficiency increased with increase in adsorbent dose up to a particular dosage (2 g) above which the efficiency reduced. An increase in biomass dosage generally increases the amount of biosorbed metal ion because of an increase in surface area of the biosorbent, which consequently increases the number of available binding sites [33]. However, adsorption capacity qe of Cd2+ decreases continuosly up to a particular dosage (1.5 g) above which efficiency increased slightly as the biosorbent was increased to 2 g and later decreased again as the biomass dosage increases to 2.5 g. This reduction in metal uptake with increasing biomass dose can be attributed to a relative amount of metal ions in solution with respect to available binding sites [34,35].
Effect of contact time
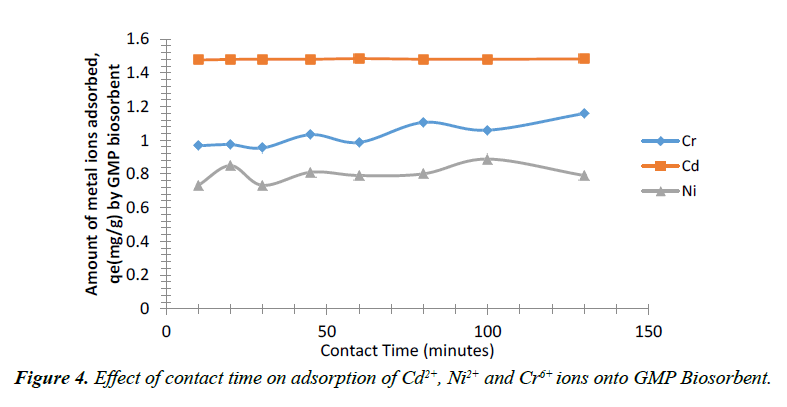
The effect of contact time on biosorption of Cr6+, Cd2+ and Ni2+ ions by GMP are shown in Figure 4. The rate of metal uptake was rapid within different time of 90 min, 60 min and 90 min respectively for Cr6+, Cd2+ and Ni2+ ions respectively. The observed fast metal ions uptake is consistent with biosorption of metal involving non-energy mediated reactions, where metal removal from solutions is purely physico-chemical interaction between biomass and metal solution. This fast metal uptake from solution indicates that binding might have resulted from interaction with functional groups on the cell wall of the biosorbent rather than diffusion through the cell wall of the biomass. Additional increase in time after the optimum time of each metal ion resulted in alternating decrease and increase in the amount of metal ions adsorbed. This implied that the active sites on the adsorbent were exhausted and further agitation resulted in desorption or the adsorption sites became saturated to maximum uptake capacity. The adsorptive capacities of Cr6+, Cd2+ and Ni2+ ions uptake by GMP at equilibrium were 1.034 mg/g, 1.485 mg/g, and 0.849 mg/g corresponding to a percentage adsorption of about 68.9%, 99.0% and 56.6% at 45, 60 and 20 min contact time. Similar trends was observed for the biosorption of Cd2+ and Cr+6 ions [26,36]. Nuhoglu and Malkoc [37] reported similar trend for Ni2+ ions adsorption which occurred in two steps; an initial fast step which lasted for about 60 min followed by as lower second phase which continued until the end of experimental period.
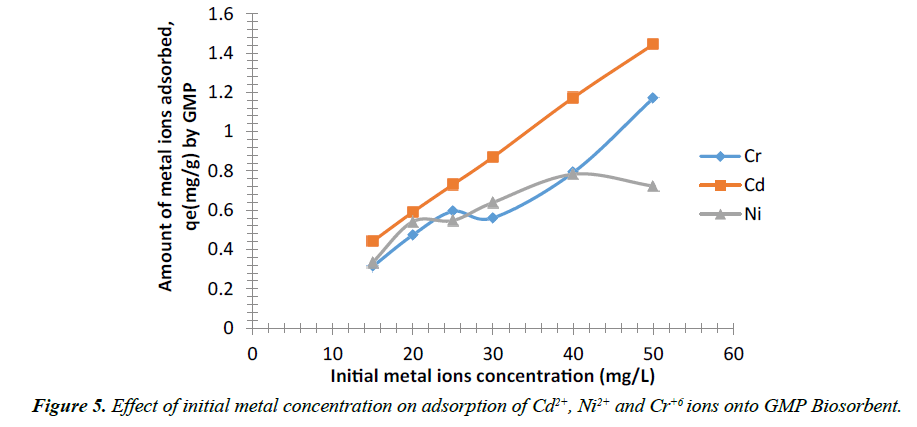
Effect of initial metal ion concentration
The result of the effect of initial metal ion concentration shows the relationship between concentrations and the amount of metal ion adsorbed at fixed adsorbent dosage (2 g). Increase in the initial metal ion concentration led to increase in amount of metal ion adsorbed. The experimental data illustrating the effect of initial metal ion concentration on rate of metal uptake of Cd2+, Ni2+ and Cr6+ ions are presented in Figure 5 respectively. The result showed a similar trend for Cr6+ and Ni2+ ions in their removal by the biosorbent mass as initial metal ion concentration increases from 15-25 mg/l, the amount adsorbed increases from 1.314 mg/g to 1.594 mg/g and 1.334 mg/g to 1.585 mg/g respectively after which a slight decrease was observed but a progressive increase was seen in the amount of Cd2+ adsorbed from 0.441 mg/g to 1.445 mg/g as the initial metal ion concentration increases from 15 to 50 mg/L. The trend of adsorption was Cd+2 > Cr6+ >Ni+2. It is generally expected that as the concentration of the adsorbate increases, the metal ions sequestered should increase according to Okeimen and Onyenkpa [38] and Elaigwu et al. [39].
This adsorption characteristic indicates that surface saturation was dependent on the initial metal ion concentrations. At low concentrations of these metal ions solution, adsorption sites took up the available metal more quickly. However, at higher concentration, metal ions need to diffuse to the biomass surface by intra-particle diffusion and greatly hydrolyzed ion will diffuse at a lower rate [40].
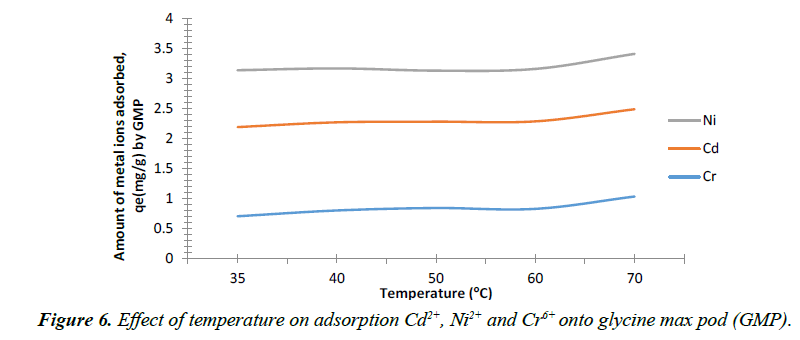
Effect of temperature
The influence of temperature on the adsorption of Cd2+, Ni2+ and Cr+6 onto GMP are shown in Figure 6. Adsorption of Cr+6 onto the biomass increased from 0.703 mg/g to 0.841 mg/g as the temperature rises from 35°C to 50°C. The increase in adsorption efficiency with rise in temperature may be an indication of chemisorption process [41]. However, the biosorption of Cd2+and Ni2+ ion onto GMP showed similar trend, adsorption decreases slightly from 1.490 mg/g to 1.444 mg/g for Cd2+ and 0.950 mg/g to 0.852 mg/g for Ni2+ as temperature rises from 35°C to 50°C and increased from 1.444 mg/g to 1.462 mg/g for Cr6+ and 0.852 mg/g to 0.877 mg/g for Ni2+ and Cr6+ with further increase in temperature from 50°C to 60°C. Similar trend was also reported for Cd2+ onto cocoa pod by Ahmet and Mustafa [42]. This decrease in the amount of Cd2+ uptake may be attributed to many parameters such as the relative increase in the dissociation tendency of the Cd2+ ions from the solid phase to the bulk phase; deactivating the biosorbent or destroying some active sites on the biosorbent surface due to bond ruptures. It may also be due to the weakness of biosorptive forces between the active sites of the biosorbent and the sorbate species and between the adjacent molecules of sorbed phase at such temperature [42].
Isotherms of the adsorption process
The results obtained from the biosorption of Cr6+ , Cd2+ and Ni2+ ions on GMP were tested using Langmuir, Freundlich and isotherms to describe the equilibrium between the metal ions sorbed on the biomass as shown in Table 1. The Langmuir isotherm showed good correlation for the adsorption of Cd2+, Ni2+ and Cr6+ ions onto GMP with a regression R2 > 0.98. This indicates that Cd2+, Ni2+ and Cr6+ ions do not form a monolayer coverage over the interface between the adsorbent GMP. The equilibrium parameter RL often referred to as dimensionless separation factor was the calculated for the adsorption of the metal ions on GMP [43,44].
| Adsorbents | Cr6+ | Cd2+ | Ni2+ |
|---|---|---|---|
| Langmuir | |||
| KL (L mg-1) | 0.623 | 0.316 | 0.174 |
| qmax(mg/g) | 24.12 | 1.702 | 1.224 |
| R2 | 0.989 | 0.9686 | 0.9923 |
| KR | 0.765 | 0.402 | 0.531 |
| Freundlich | |||
| KF (Lg-1) | 7.102 | 1.047 | 4.282 |
| 1/n | 0.759 | 0.563 | 0.403 |
| R2 | 0.674 | 0.822 | 0.747 |
| qexp. (mg/g) | 1.0335 | 1.485 | 0.849 |
| Dubinin-Redushkevich | |||
| qRD (mg/g) | 22.94 | 22.8 | 28.31 |
| β (kJ/mol2) | 4.93 x 10-7 | 6.21 x 10-7 | 9.14 x 10-7 |
| E (kJ/mol) | 1.007 | 0.707 | 0.739 |
| R2 | 0.897 | 0.905 | 0.913 |
Table 1: Isotherm constants of two-parameter models for Cr6+, Cd2+ and Ni2+ biosorption by GMP.
Where KL is the Langmuir constant obtained from the initial Langmuir plot, and Ci is the initial concentration (mg/L).The values of RL obtained from Equation 4 all fall within 0 and 1 (0< RL<1), indicating that the adsorption process is favorable [21].
The adsorption of Cr+6, Cd2+ and Ni2+ ions onto GMP was equally described by the Freundlich model. The Freundlich adsorption isotherm supports the heterogeneity of the surface and the exponential distribution of the sites and their energies. The KF and 1/n values obtained for adsorption of Cd2+, Ni2+ and Cr6+ ions were (1.047, 4.282 and 7.102) and (0.759, 0.563, 0.403) respectively. 1/n is an empirical parameter relating to the biosorption intensity and varies with the heterogeneity of the material. Values between 0 to 1 for Cd2+, Ni2+ and Cr6+ ions indicated that biosorption of the metal onto GMP was favorable at studied conditions.
The D-R isotherm assumes a fixed volume or ‘adsorption space’ where adsorption takes place. It also describes heterogeneity of adsorption energies within this space independent of temperature. It was observed that the D-R isotherm gave a good fit for the adsorption of both metals to both biomasses when compared with Langmuir, Freundlich. The E values obtained of the biosorption processes were all in the range of ion exchange reactions [16].
Kinetics of biosorption process
Kinetic data obtained in this study was fitted to Equations 5 t o 8. Kinetic parameters obtained are shown in Table 2. The results obtained indicate that only the pseudo-second order equation was able to satisfactorily fit the kinetic data for GMP on Cr6+, Cd2+ and Ni2+ ions in the whole data range. R2 values and are very high (0.9998, 1.0, 0.9919) respectively. Also, the theoretical qe2cal values were closer to the experimental qe2exp values. In this model, the rate - limiting step is a biosorption mechanism involving chemisorption, where metal removal from solution is due to physicochemical interactions between biomass and metal solution [21]. According to the pseudo- second order kinetic model, the rate of biosorption reaction non-linearly decreases with time [45].
| Adsorbents | Cr6+ | Cd2+ | Ni2+ |
|---|---|---|---|
| Pseudo first order | |||
| K1 (min-1) | -5.5×10-3 | 8.01 × 10-3 | 6.04×10-3 |
| qcal (mg/g) | 0.027 | 0.0013 | 0.094 |
| R2 | 0.6702 | 0.0285 | 0.2915 |
| Pseudo second order | |||
| K2 (min-1) | 1.59 × 10-3 | 1.77 × 101 | 1.8 × 10-1 |
| qcal (mg/g) | 16.37 | 28.28 | 32.54 |
| R2 | 0.9998 | 1 | 0.9919 |
| qexp. (mg/g) | 16.85 | 28.5 | 34.93 |
| Elovich | |||
| a (mg/g min) | 8.44 x 1010 | 1.55 x 1019 | 1.97 x 1011 |
| b (g/mg) | 0.979 | 1.174 | 1.231 |
| R2 | 0.9932 | 0.9354 | 0.961 |
| Intraparticle diffusion | |||
| kp (mg/g min0.5) | 6.781 x 10-1 | 5.862 x 10-1 | 1.36 x 10-1 |
| R2 | 0.9756 | 0.9209 | 0.9195 |
Table 2: Kinetic model parameters for Cr6+, Cd2+ and Ni2+ biosorption by GMP.
The pseudo first order kinetic model did not appropriately describe the kinetic data in the whole data range. The other three kinetic models (intra particle diffusion model, Elovich model and the fractional power model) also did not give satisfactory fits when fitted for their applicability to the kinetic data. However, it was observed that the biosorption process presented by these models comprised of two phases suggesting their applicability to a particular period of the adsorption process. The three models were then tested on the two phases presented. It was observed that all data obtained before the saturation point of the biomass followed the assumptions of these models as indicated by their regression values. A similar method had been adopted for describing kinetic data for biosorption [46].
Conclusion
The potential application of Glycine max pod in removal of Cd2+ and Ni2+ and Cr6+ from wastewater effluents was tested in present study using batch adsorption process. Adsorption characteristics of Cd2+ and Ni2+ and Cr6+ onto GMP were found to be influenced by initial pH of aqueous solution, contact time, initial metal ions concentration, temperature, and GMP dosage. Perfect metal uptake efficiencies were obtained under operating conditions such that for Cd2+ and Ni2+ ions by Keeping he experimental conditions as follows; initial pH: 5.0 and 6.0, Contact time Cd2+/Ni2+: 90/60 min, GMP dosage: 2 g and initial Cd2+/Ni2+ concentration: 25/50 mgL-1, nearly100% removal efficiency was obtained. And for Cr+6 ions, the removal efficiency was reached to79%, while the experimental conditions were as follows; initial pH: 3.0, contact time: 90min, GMP concentration: 2.0g and initial Cr6+ concentration: 50 mgL-1 [47-50].
Kinetic models evaluated include Intraparticle diffusion model, Elovich model and pseudo-first and second order models. While the kinetic data for all the adsorption experiments satisfactorily fitted to the pseudo-second order model, the assumptions of the Elovich and intra-particle diffusion were apparently important before and after the saturation points of the biosorbents. The fast kinetics of the adsorption process and the high percentage removal from concentrated solutions under optimized conditions are useful properties of the adsorbent which can be developed by modification.
Equilibrium adsorption of all the metals could not be described by the simple Langmuir and Freundlich models alone. However, a combination of other two-parameter equilibrium models suggestively describes the nature of the adsorption processes. The R-D model, amongst the evaluated models gave the best fit for all the adsorption processes studied.
In the view of these results, it can be concluded that the GMP can be utilized as a low cost and effective biosorbent in removal of Cd2+ and Ni2+ and Cr6+ ions from wastewater and industrial effluents.
References
- Dang VBH, Doan HD, Dang-Vu T, et al. Equilibrium and kinetics of Cu(II) adsorption by Rhizopus arrhizus, Cladosporium resinae and Penicillium italicum. Applied Microbiology and Biotechnology. 2009;26:84-90.
- Guiqiu C, Guangming Z, Lin T, et al. Cadmium removal from simulated wastewater to biomass byproduct of Lentinus sedodes. Bioresource Technology. 2008;99:7034-40.
- Cheung CW, Porter JF, Mckay G. Sorption kinetic analysis for the removal of cadmium ions from effluents using bone char. Water Resource. 2001;35:605-12.
- Malkoc E, Nuhoglu Y. Investigations of nickel (II) removal from aqueous solutions using tea factory waste. Journal of Hazardous Material. 2005;127:120-28.
- Ofomaja AE, Ho YS. Effect of temperature and pH on methyl violet biosorption by Mansonia wood sawdust. Bioresource Technology. 2007;9(9):5411-17.
- Babel S, Kurniawan TA. Low cost adsorbents for heavy metal uptake from contaminated water: A review. Journal of hazardous marerials. 2003;97(1-3):219-43.
- Patterson JW. Industrial Wastewater Treatment Technology, second ed. Butterworth Heinemann. ISBN-13: 978-0409900026. 1985.
- Donmez GC, Aksu Z. Removal of Cr (VI) from saline waste waters by Dunaliella species. Process Biochemistry. 2002;38:757-62.
- Dakiky M, Khamis M, Manassra A, et al. Selective adsorption of Cr(VI) in industrial wastewater using low-cost abundantly available adsorbents. Advance Environmental Resources. 2002;6:533-40.
- http://www.unep.org/urbanenvironment
- Park D, Yun YS, Park JM. Studies of hexavalent chromium biosorption by chemically treated biomass of Ecklonia Sp. Chemosphere. 2005;60(10):1356-64.
- Gupta VK, Jain CK, Ali I, et al. Removal of cadmium ion: Metal Pollution in the Aquatic Environment. New York; Springer. 2007;97-269.
- Sears GW. Determination of speci?c surface area of colloidal silica by titration with sodium hydroxide, Anal. Chem. 1956;28:1981-83.
- Iler RK. The Chemistry of Silica, John Wiley & Sons, New York. 1979:203-206.
- Olu-Owolabi BI, Diagboya PN, Ebaddan WC. Mechanism of Pb (II) removal from aqueous solution using a non-living moss biomass. Chemical Engineering Journal. 2012a;195:270-75.
- Unuabonah EI, Adebowale KO, Dawodu FA. Equilibrium, Kinetic and sorber design studies on the adsorption of Aniline blue dye by sodium tetraborate-modi?ed Kaolinite clay adsorbent. Journal of Hazardous Materials. 2008;5:781-95.
- Sposito G. The Chemistry of Soils, Oxford University Press, New York. 1989.
- Dubinin MM, Radushkevich DV. Equation of characteristic curve of activated charcoal. Proceed. Acad. Sci. USSR. 1947;55:331-33.
- Langergren S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetenskapakad. hand lingnaar, Band. 1898;24(4):1-39.
- Ho YS, Mckay G. Pseudo-second or de model for sorption processes. Process Biochemistry. 1999;34:451-65.
- Olu-Owolabi B, Oputu OU, Adebowale KO, et al. Biosorption of Cd2+ and Pb2+ ions onto mango stone and cocoa pod waste: Kinetic and equilibrium studies. Scientific Research and Essays. 2012b;7(15):1614-29.
- Ofomaja AE, HO YS, Unuabonah IE. Effect of pH on cadmium biosorption by coconut copra meal. Journal of Hazardous Materials. 2009;13(2):356-62.
- Iqbal M, Saeed A, Zafar SI. FTIR spectroscopic, kinetics and adsorption isotherms modeling, ion exchange, and EDX analysis for understanding the mechanism of Cd2+ and Pb2+ removal by mango peel waste. Journal of Hazardous Material. 2009;164:161-71.
- Hanif MA, Nadeem R, Bhatti HN, et al. Ni(II) biosorption by Cassia fistula (golden Shower) biomass. Journal of Hazardous Materials. 2007;139:345-55.
- Zheng JC, Feng HM, Lam MH, et al. Removalof Cu(II) in aqueous media by biosorption using water hyacinth roots as a biosorbent material. Journal of Hazardous Materials. 2009;17(1):780-85.
- Jimoh T, Buoro AT, Muriana M. Utilization of Blighia sapida (Akee apple) pod in the removal of lead, cadmium and cobalt ions from aqueous solution. Journal of Environmental Chemistry and Ecotoxicology. 2012;4(10):178-87.
- Babalola JO, Overah LC, Babarinde A, et al. Kinetic, Equilibrium and thermodynamic studies on the biosorption of Cd(II) from aqueous solutions by the leaf biomass of Calotropis procera- ‘Sodom apple’. Journal of Applied Science Environment Management. 2011;15(4):607-15.
- Egila JN, Dauda BEN, Jimoh T. Biosorptive removal of cobalt (II) ions from aqueous solution by Amaranthus hydridus L. stalk wastes. Africa Journal of Biotechnology. 2010;9(8):8192-98.
- Shama SA, Moustafa MA, Gad MN. Removal of heavy metal from aqueous solutions by using Eichhornia erassipes. Port. Electrochimica Acta. 2010;28(2):125-33.
- Wang X, Xia S, Chen L, et al. Biosorption of Cd(II) and lead(II) ions from aqueous solutions onto dried activated sludge. Journal of Environmental Sciences. 2006;18:840-44.
- Qaiser KK, Lee CK, Low KS, et al. Removal of Cu and Pb by tartaric acid modified rice husk from aqueous Solutions. Chemosphere. 2009;50:23-28.
- Saeed A, Iqbal M, Akhtar MW. Removal and recovery of lead (II) from single and multimodal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). Journal of Hazardous Materials. 2005;117(1):65-73.
- Razmovski K, Sciban M. The kinetics of copper (II) adsorption from water by some natural materials. J. Environ. Protect. Ecol. 2003;4:728-32.
- Choi M, Jang J. Heavy metal ion adsorption onto polypyrrole-impregnated porous carbon. Journal of Colloid and Interface Science. 2008;325:287-89.
- Amuda OS, Giwa AA, Bello IA. Removal heavy metals from industrial wastewater using modified activated coconut shell carbon. Biochemical and Engineering Journal. 2007;36(2):174-81.
- Aksu Z, Isoglu IA. Removal of copper (II) ions from aqueous solution by biosorption on agricultural waste sugar beet pulp. Process Biochemical. 2005;40:3031-44.
- Nuhoglu Y, Malkoc E. Thermodynamic and kinetic studies for environmentally friendly Ni(II) biosorption using waste pomace of olive oil factory. Bioresource Technology. 2008;10:2375-80.
- Okeimen FE, Onyenkpa VU. Binding of Cadmium, Copper, Lead and Nickel ions with melon (Citrullus vulgaris) seed husk. Biology Waste. 2000;29:11-16.
- Elaigwu LA, Usman GV, Awolola GB, et al. Adsorption of Pb(II) from aqueous solution by activated carbon prepared from Cow Dung. Advance National Applied Science. 2009;3(3):442-46.
- Horsfall M, Spiff AI. Studies on the effect of pH on the sorption of Pb2+ and Cd2+ ions from aqueous solutions by Caladium bicor (wild cocoyam) biomass. Electronic Journal of Biotechnology. 2004;8(2).
- Kara M, Yuzer H, Sabah E, et al. Adsorption of cobalt from aqueous solution onto Sepiolite. Water Research. 2003;37:224-32.
- Ahmet DM, Mustafa T, Mustafa S. Biosorption of Cd(II) and Cr(III) from aqueous solution by moss (hylocomium splendens) biomass; equilibrium, kinetic and thermodynamic study. Chemical Engineering Journal. 2008;14(4):1-9.
- Adebowale KO, Unuabonah IE, Olu-Owolabi BI. The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. Journal of Hazardous material. 2006;134:130-39.
- Ahalya N, Ramachandra TV, Kanamadi RD. Biosorption of heavy metals in aqueous solution. Research Journal of Chemistry and Environment. 2003;7:71-78.
- Alluri HK, Ronda SR, Sattalluri VS, et al. Biosorption: An eco-friendly alternative for heavy metal removal. African Journal of Biotechnology. 2007;6(25):2924-31.
- El-Sayed GO, Dessouki HA, Ibrahim SS. Biosorption of Ni(II) and Cd(II) ions from aqueous solutions onto rice straw. Chemical Sciences Journal. 2010;2(10):1-9.
- Akgerman A, Zardkoohi M. Adsorption of phenolic compounds on fly ash. Journal of chemistry and Engineering. 1996;4:185-91.
- Duygu Ozdes, Celal Duran, Hasan Basri Senturk. Adsorptive removal of Cd(II) and Pb(II) ions from aqueous solutions by using Turkish illitic clay. Journal of Environmental Management. 2011;92:3082-90.
- Freundlich H. Ueber die Adsorption. Loesungen Zeitsch Physical chemistry. 1907;57:385-470.
- Langmuir I. The adsorption of gases on the plane surfaces of glass, mica and platinium. Journal of American chemical society. 1918;40:1361-1403.