- Biomedical Research (2015) Volume 26, Issue 2
The Effect of Gold Nanoparticle on Luteinizing Hormone, Follicle Stimulating Hormone, Testosterone and Testis In Male Rat.
Mohammadreza Behnammorshedi*, Habibollah Nazem, Masoud Saleh moghadam
Department of Biology, Payame Noor University, I.R. of Iran
- Corresponding Author:
- Mohammadreza Behnammorshedi
Department of Biology Payame Noor University I.R. of Iran
Accepted date: February 09, 2015
Abstract
Nanoparticles for their special and unique features are widely used in different industries such as medicine and pharmaceutical. In addition to positive aspects of gold nanoparticles, its toxicity in environment is unavoidable. This study was conducted on 40 Wistar rat, divided in 5 groups including a control, a sham and three other experimental groups. The control group was treated daily with 0.5 cc saline solution, the sham group had food and water, and three other groups received intraperitoneal injection of 0.5 cc gold nanoparticles at different doses (100, 50, 25 ppm). After 10 days of treatment, animals were anesthetized; blood samples were collected from the heart and luteinizing hormone (LH), follicle–stimulating hormone (FSH), and testosterone (T) were measured. Testes were removed and preserved in formaldehyde (10%) solution. For statistical analysis, the analysis of variance (ANOVA) and Dunnett test were performed by SPSS software, version19. Results showed that gold nanoparticles have significant effect on hormones in concentrations of 100 ppm. LH, FSH and testosterone were significantly increased compared to (P<0.05). Seminiferous tubular degeneration was also observed in the testes. These findings suggest that gold nanoparticles may cause an increase in hormones in female and in male with subsequent increase in sterility.
Keywords
Zinc oxide nanoparticle, testosterone, LH, FSH
Introduction
Nanomedicine has wide range of applications. One of the most important difficulties of nano medicine is to understand its effectiveness on environment and also to determine the possible toxicity of nanoscale materials [1]. Nano particles can be beneficial or be damaging at the same time. Materials could be toxic and hazardous when they are converted into nano form [2]. Additionally, the small size of nanoparticles allows them to overcome the defense barriers of the body without facing any obstacles [3]. Nanoparticles can be hazardous to health for two reasons; firstly, the nanoparticles can easily be absorbed through skin and epithelial cells and secondly, new and unknown nanoparticles exert toxic effects. Nano particles are so small that they can penetrate cell membrane. Larger particles could carry a foreign substance between “DNA” strands [4]. Nowadays, different kinds of coatings such as albumin, dextran, polyethylene glycol, polyethylene oxide, aspartic acid, etc., are being used to exploit the beneficial applications of nanoparticles in biological systems. Using of such coatings can be resulted in the stability of nanoparticles in biological solutions, in circulation, and their distribution in tissues. Then can also facilitate entering nanoparticles into cells and reduce their toxic effects [5,6]. Gold nanoparticles range from 3 to 100 nanometers. These nanoparticles can be used as potential detectors in designing of biosensors. The methods, which might be used are optical absorption spectroscopy, florescence, Raman scattering, magnetic force and electric current methods.
These particles can be used to detect DNA, proteins, microorganisms, etc. Detection of DNA using Au nanoparticles is 10 times more sensitive and 100000 times more specific than the conventional genomic detection systems [7]. Goodman showed that cationic gold nanoparticles are moderately toxic, whereas anionic gold nanoparticles are quite non-toxic [8], while gold nanoparticles of diameter 1.4 nm are highly toxic [9], AU15MS compounds are quite non-toxic. Particles of 1-2 nm sizes have high toxicity for all gold nanoparticles compounds and particles with the sizes larger than 15 nm are moderately non-toxic. The nanoparticles centrifuged during a 24 hours period at the temperature of 4°C produced a dark red solution. Nanoparticles of 12, 4, 18 nm diameter are non-toxic to treat leukemia cells [11]. The aim of present study was to investigate the effects of gold nanoparticles toxicity on the changes in concentrations of luteinizing hormone (LH), follicle-stimulating hormone (FSH) and testosterone (TSS) in the blood and testes.
Materials and Methods
Materials
The materials used in this study consisted of gold nanoparticles, acetone, and ethanol. Chloroform which was used for the preparation of nanoparticles and other materials used in this study included saline, ketamine, hematoxylin, eosin (Merck, Germany), entellan glue (Merck, Germany), xylol (Part-Teb and Teb-Al-Sadiq, IR.Iran), methanol (Part-Teb and Teb-Al-Sadiq, IR.Iran), formaldehyde (Part-Teb and Teb-Al-Sadiq, IR.Iran) and experimental kit (DB52181, Germany).
Apparatus
The device used in this study included XRTEM (JEM- 200CX) and optical microscope (OLYMPUS CX 21 FS1, Turkey and Japan), centrifuge (Hettich,ununiversal), microtome (LIETZ 1512, Germany), tissue bath (BI, Germany), four1 (BEHZAD NOVIN, IR.Iran), and optical microscope (OLYMPUS CX 21, Turkey and Japan).
Synthesis of gold nanoparticles
Trisodium citrate, a reducing agent was used as a nucleation factor (Turkevich method). Usually a mixture of HAuCl3 is heated to boil, stirred and then the aqueous solution of sodium citrate is added to it. The solution is kept at boiling temperature. Following the addition of sodium citrate the purple color appeared, which is becoming red. A step called delay or induction phase is occurred before the change of color, which resulted in the formation of precursors such as actone decarboxylic acid that is a product of the oxidation of citrate.
Animals
Suspension of purchased gold nanoparticles was administered to rats with different doses via oral gavage. Fourty adult Wistar rats weighing 250-200 grams at the age of 8 weeks were used to perform the test. The animals were purchased from Pasteur Institute of Iran, Tehran, IR Iran and divided into 5 groups including controls and three experimental groups. Eight rats were included in each group and all groups were kept in polypropylene cages with sawdust-covered floors and water bottles. Animals were kept under controlled conditions of temperature (22 ± 10 °C), humidity (60 ± 10%), and light (12 hours brightness, 12 hours darkness), with free access to water and food (Figure 1-2). All the rats were kept in the nest with similar conditions for 2 weeks prior to start of the experiments for adaptiation to their environment and diet. All procedures followed were in accordance with the ethical committee. Treated animals were marked by signs and were fed by oral gavage for10 days at the doses of 100, 50, 25 ppm and grouped accordingly. To test the hormones by tumor markers, blood sampling was done after 10 days of treatment. Blood samples were taken from the heart. The samples taken from the animals were placed in the special centrifuge tubes. Testes were removed and placed in a formaldehyde (10%) solution and sent to the laboratory for hematoxylin-eosin staining.
Data analyses
Data obtained from Electrochemiluminesence device was saved and analyzed using SPSS software, version19. Analysis of variance (ANOVA) test was performed by SPSS, and then the outputs were transferred to the Excel program. The results were presented as mean and standard deviation (SD). The data were normally distributed, therefore, repeated measures ANOVA was used to compare the enzymatic results among the groups before and after the experiment. ANOVA and Dunnett tests were applied to compare the groups together in each periods of time. The Level of significance was considered less than 0.05.
Results
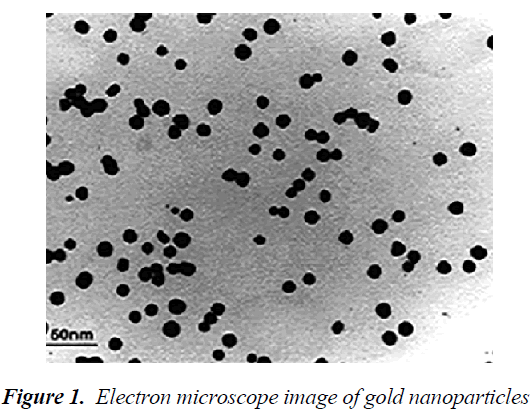
Electron microscope study of gold nanoparticles
Transmission electron microscope (TEM) is much larger than the light microscope. In this microscope, unlike the light microscope, the beam shines from top-to-bottom, however the principles of its operation are similar to the light microscope. The TEM has a long column and electron beam source is located at the top of this piston. Electron beams after passing through the sample, hit a film or a screen (which is made of fluorescent material) and causing image formation. Images of electron microscope are black and white, because some beams do not pass through the sample and create black dots. Transmission eelectron microscope (TEM) image of synthesized gold nanoparticles is shown in Figure 1. An increased surface to volume ratio of nanoparticles by reducing the size of the smaller nanoparticles could play a good role in the immobilization processes. According to the results of the TEM studies, the diameter range of 10 nm had been found for gold nanoparticles[12].
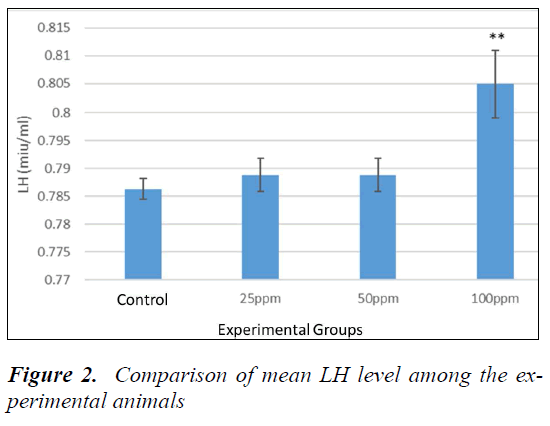
Analysis of LH
Table 1-3 show P > 0.05. However, no significant difference between sham and controls was evident. To compare the LH levels, animals all groups were compared with the control. The mean level of LH was increased in the animals receiving nanoparticles compared to the control. According to Dunnett's test for comparison, the level of LH in animals receiving 100 ppm was significant compared to the control (P = 0.004) (Fig. 2).
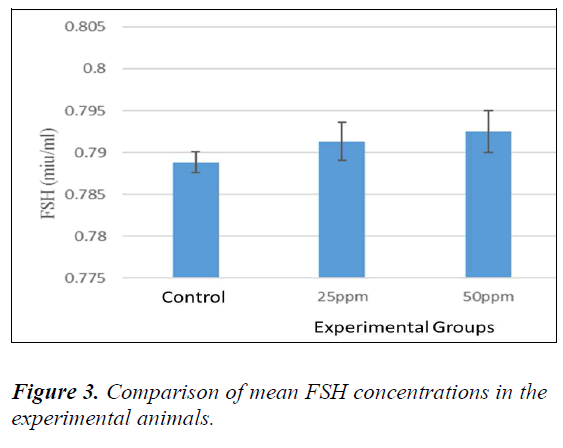
Analysis of FSH
No significant difference was evident between sham and controls (P > 0.05). Therefore, to compare the FSH levels in the serum, all the animals were compared with controls. The mean level of FSH was increased in animals receiving nanoparticles in comparison with the controls. Table 4-3 ahow comparison among groups (Dunnett’s test). The level of FSH in the animals receiving 100 ppm was found to be significant compared to the controls (P = 0.036) (Fig. 3).
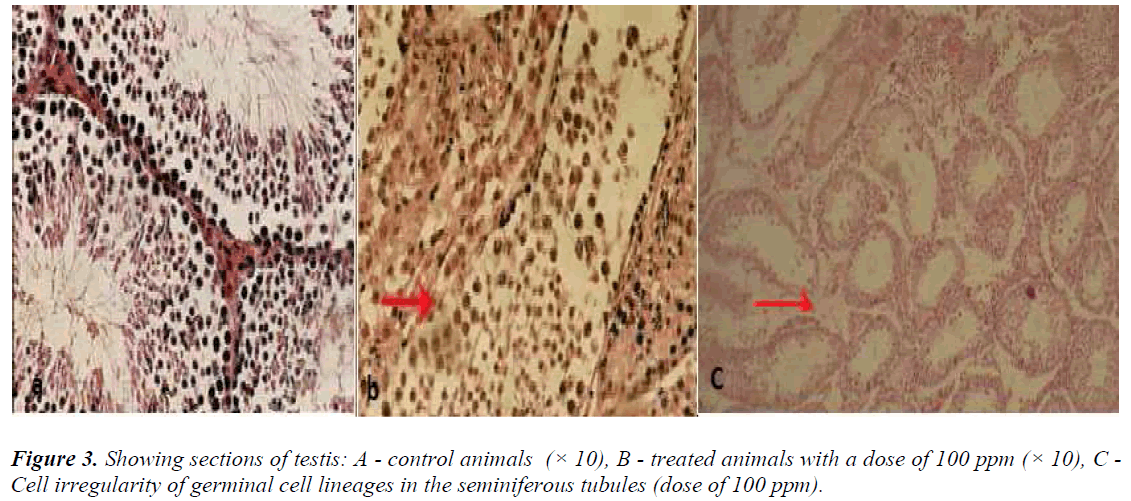
Testis
In the control animals, no detectable changes were seen in the interstitial cells of Leydig, seminiferous tubules, and testicular cell lineages. In the animals, which received nanoparticles at a dose of 100 ppm, degeneration of seminiferous tubules was visible (Figure 3b).
Analysis of testosterone (T)
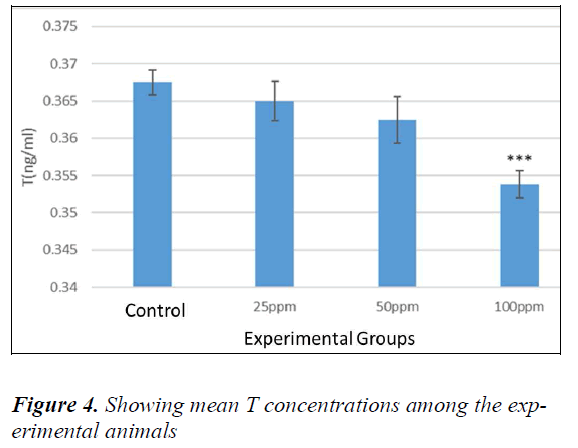
No significant difference was evident in animals between the sham and controls (P > 0.05). T levels in the serum, animals of all the groups were compared with the controls. The mean level of T was found to be decreased with an increased dose of nanoparticles. The mean levels of T were decreased in the animals those received nanoparticles in comparison to the controls. According to Dunnett's test for comparison among groups, the level of T in the animals receiving 100 ppm was found to be significant compared to the control. (P = 0.001) (Fig. 4).
Discussion
The science of nanotechnology is based on the fact that materials reduced to the nanoscale possess special and extraordinary properties [24]. When the bulk materials are converted into smaller structures or dimensions (such as length, width or thickness) in the range of nanometers or less, unique particles with unpredictable properties could be significantly different compared to the bulk material characteristics [13]. In a study conducted by Rezaei et al., (2012), the effects of silver nanoparticles on LH, FSH and testosterone in the male rats was investigated. The doses used were 200, 100, 50, 25 mg per kg body weight per day orally for 28 days. In our study, significant changes were not found in hormone concentrations up to 100 mg; however, a significant decrease in testosterone concentration was evident at higher doses (i.e. 200 mg) in comparison with controls, which could be caused by inhibitory effect of silver nanoparticles on the function of cells that produce testosterone. Results of out study also showed that silver nanoparticles decreased the levels of LH, FSH and testosterone in male rats [14], Silver nanoparticles also reduced Leydig cell mitochondrial secretory activity. Furthermore, silver nanoparticles caused an increase in oxygen free radicals, such as superoxidase and increased oxidation of molecules such as proteins [15].
Another study investigated the effect of silver nanoparticles consumption at concentrations of 95, 65, 35, 20, and 5 ppm instead of drinking water. After 3 and 6 months, 3 animals from each group were selected and the levels of sex hormones were evaluated. It was found that the level of testosterone increased with “testicle hyperactivity”. It was also found that nanoparticles could cause alterations in endocrine glands function [16] with decrease in the level of testosterone. Our study also determined the effects of iron oxide nanoparticles on the number and motility of sperm cells. Seventy-five (75) male mice were divided into 5 groups with 15 mice each in control and 4 expremental groups. Iron oxide nanoparticles dissolved in distilled water at doses of 40, 20, 10 and 5 mg / kg and injected intraperitoneally. At the end of the experimental period; spermatogenesis, sperm samples from the tail of the epididymedes were studies. Percentages of motile and immotile spermatozoa, as well as sperm cells count were made for each group of mice. Based on the results of this study it could be suggested that the iron oxide nanoparticles have a negative effect on sperm count and motility and this negative effect is directly linked with higher doses of iron oxide nanoparticles [17]. Carlson et al., (2008) found that the nanoparticles can affect the leydig cell mitochondrial activity and thus reduce its secretion activity. In addition, nanoparticles also caused an increase in oxygen free radicals, such as superoxidase and oxidation of molecules such as proteins [18], eventually reduce the leydig cell counts and decrease in the production of testosterone, which is consistent with our findings. Another assumption is that the nanoparticles could affect the gene expression of the protein that is involved in the transport of cholesterol into the inner membrane of mitochondria and increased the synthesis of steroids. It is also possible that nanoparticles by reducing the gene expression of the mitochondrial membrane protein Star, inhibit the cholesterol transportation into the inner membrane of mitochondria, and eventually inhibit the conversion of cholesterol to pregnenolone and reduced the level of testosterone. Karpenko (2013) studied toxic effects of cerium oxide nanoparticles on sex hormones and concluded that nanoparticle reduced glandular and testosterone secretion, which was increased after 70 days of treatment [19]. Our study also showed that gold nanoparticles reduced the level of testosterone.
Nonoparticles can penetrate the blood-testis barrier and adversely affect the sperm cells [20]. Researchers showed that titanium oxide nanoparticles might produce infertile sperms and abnormal Leydig cells [21]. Nanoparticles could also accumulate in the Leydig, sertoli, and spermatogenic cells [22].
Conclusion
According to the findings, it could be concluded that the negative effects of nanoparticles are greater than the positive effects, but in general, regardless of the dual effects, the mechanism of their action is to some extent similar. Nanoparticles also showed significant negative effects on the body. At the cellular level, the free radicals is increased and caused significant tissue damage. Our study showed that different doses of gold nanoparticles exert negative impact on testicular hormone secretion . By generating free radicals and reactive oxygen species, nanoparticles caused a considerable damage to the testis.
References
- Fiorito S, Serafino A, Andreola F, Togna A, Togna G. Toxicity and biocompatibility of carbon nanoparticles. J Nanosci Nanotechnol. 2006; 6(3): 591-599.
- Fiorito S, Serafino A, Andreola F, Bernier P. Effects of fullerenes and single-wall carbon nanotubes on murine and human macrophages. Carbon. 2006; 44(6): 1100- 1105.
- Reddy LS, Nisha MM, Joice M, Shilpa PN.” Antimicrobial activity of zinc oxide (ZnO) nanoparticle against Klebsiella pneumonia” Pharm Biol. 2014; 15: 1.
- Mital GS, Manoj T. “A review of TiO2 nanoparticles” Chinese Sci Bull. 2011; 56: 1639.
- Chen Z, Meng H, Xing G, Chen C, Zhao Y, Jia G, et al. “Acute toxicological effects of copper nanoparticles in vivo” Toxicology Letters.163(2006) 109.
- Jun, S.K., Tae, J.Y., Kyeong, N.Y., Byung, G.K., Sung, J.P., Hyun, W.K., Kee, H.L., Seung, B.P., Jin, K.L., and Myung, H.C., “Toxicity and Tissue Distribution of Magnetic Nanoparticles in Mice” Toxicological Sciences. 89(2006) 338.
- Vila A, Sanchez A, Tobio M, Calvo P, Alonso M. Design of biodegradable particles for protein delivery. Journal of Controlled Release. 2002; 78(1): 15-24.
- Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjugate chemistry. 2004; 15(4): 897-900.
- Tsoli M, Kuhn H, Brandau W, Esche H, Schmid G. Cellular uptake and toxicity of Au55 clusters. Small. 2005; 1 (8-9): 811-814.
- Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: what we have learned so far? Journal of Nanoparticle Research. 2010; 12(7): 2313- 2333.
- Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005; 1(3): 325-327.
- Ali Karami, Mohammadreza Behnammorshedi, Saeedeh Pouri, Masoud Negahdary, Marziyeh Ajdary. Single-Walled Carbon Nanotube and Hemoglobin used in a Dopamine Biosensor. Int. J. Electrochem. Sci 2014; 9: 8367-8379
- Afkhami Ardakani Mohammad, Rezaei Zarchi Saeed, Golzadeh Jalal, Shirband Ali. Effect of iron oxide nanoparticles on the testis and testosterone in male rats. First National Conference on Nano Science and Technology. 2010 [In Persian]
- Rezaei Zarchi Saeed, Ramezani Mina, Nasri Sima, Houshyar Leyla. Evaluation of damaging effects on ovaries in rats orally treated with silver nanoparticles. First National Conference on Nano Science and Technology. 2010 [In Persian]
- Phalen RF, marrow PE. Experimental inhalation of metallic silver. Health Phys 1973; 24: 509-518
- Roshanayee Kambiz, Razavian Seyyed Mohammad Hosein, Ahmadi Ramesh, Heidariyeh Nasrin, Massayeemanesh Mohammad Baqer. The Effect of Silvernano Oral Consumption on some Hormonal, Hematological and Urine Parameters of Vistar Rats. Qom University of Medical Sciences Journal 2012. 6(3): 65-70 [In Persian]
- Nasri Sima, Rezaei Zarchi Saeed, Kerishchi Khiabani Parisa, Sadeqi Safoura. Effects of iron oxide nanoparticles on the number and motility of sperm in male mice. First National Conference on Nano Science and Technology. 2012 [In Persian]
- Carlson C, Hussain SM, Schrand AM, et al. unique cellular interaction of silver nanoparticles: sizedependent generation of reactive oxygen species. J Phys Chem B, 2008; 112(43): 13608-13619.
- Karpenko N.A, Malukin Yu.V, Koreneva E.M, Klochkov V.K, Kavok N.S, Smolenko N.P, Pochernyaeva S.S. The Effects of Chronic Intake of Cerium Dioxide or Gadolinium Ortovanadate Nanoparticles in Aging Male Rats. Nanomedicine, 2013; 2(4): 4.
- Cohen G, Spina MB. Deprenyl suppresses the oxidant stress associated with increased dopamine turnover. Annals of neurology. 1989; 26(5): 689-690.
- Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006; 311(5761): 622-627.
- Ema M, Kobayashi N, Naya M, Hanai S, Nakanishi J. Reproductive and developmental toxicity studies of manufactured nanomaterials. Reproductive Toxicology. 2010; 30(3): 343-352.