Review Article - Current Pediatric Research (2021) Volume 25, Issue 8
The effect of different decontaminants on green vegetable foods collected from Iraqi stores.
Hadeel K Turki1, Zahraa Muhsen M Ali2, Ali H Saleem1*
1Department of Dentistry, Al-Rafidain University College, Baghdad, Iraq
2Department of Medical Laboratory Technique, Al-Rafidain University College, Baghdad, Iraq
- Corresponding Author:
- Ali H Saleem
Department of Dentistry
Al-Rafida in University College
Baghdad
Iraq
E-mail: abujamaljameel@gmail.com
Accepted date: 28th August, 2021
Abstract
Green vegetable foods are highly contaminated with bacteria and in order to reduce viable bacterial count in this important type of foods and make it less harmful; this research used different types of decontaminants in certain acceptable concentrations. Twenty five bundles of green vegetable foods had been collected from vegetable shops and peddlers in Baghdad, samples had been washed three times and treated with 0.1% potassium permanganate, 10% sodium chloride (table salt), 5% acetic acid (apple vinegar), and 2 Parts Per Million (PPM) of sodium hypochlorite separately, then the soaked water had been inoculated on nutrient agar and incubated for a viable bacterial count. It had been found that these substances have clear effect in bacterial reduction and this may help in reducing the harmful effect of pathogenic ones for human health 0.1% potassium permanganate was the most effective decontaminant, followed by 5% acetic acid, 10% table salt, while 2 ppm of sodium hypochlorite exhibited less effectiveness. Using of different types of decontaminants in certain concentrations that have a lower harmful effect on health and on plant structure with green vegetable foods helps in bacterial reduction and contributes in preventing their harmful effect.
Keywords:
Decontaminants, Green vegetable foods, Bacterial viable count.
Introduction
In human daily food need, green leaves vegetable should be used as a main source for the most important vitamins and other body requirements, they play an important role in human health and metabolism in human body physiology especially when they consumed raw without exposure to heat [1]. Because of different unsafe methods of crop irrigation, contact with animals, and handling after harvesting, many types of bacteria grow on these vegetable causing contaminations leading to different types of disorders [2]. Recent studies confirm the association between fresh green leaves vegetable intake and gastrointestinal disorders, some of these disorders caused by pathogenic microorganisms, while others may be caused by toxins produced by them, these toxins may produce nervous system disorders [3].
Decontaminants should be used on green leaves vegetable in order to reduce the harmful effect of contaminant microorganisms. Substances in certain concentrations, including potassium permanganate, sodium chloride (table salt), acetic acid (apple vinegar), and sodium hypochlorite (household bleach product) usually used as decontaminants for green leaves vegetable in household. These substances should be used in certain concentrations that can give the best results with preserving the nutritional value of vegetables and with less toxic effect on human health [4-6]. Other substances may have an effect against microbial contaminants but it less used in a household, they may be used in large food factories or for ready to eat vegetables in markets [6].
Aim of the Study
The aim of this study was to find the most effective substance for decontamination of green vegetable foods in a household that makes it safe to eat with less harmful effect.
Materials and Methods
This includessample collection, preparation of samples into groups, preparation of decontaminant's concentrations, bacterial counting.
Sample collection
Samples had been collected during the period from October to December 2020 from different sites of vegetable shops and peddlers in Baghdad, a total of 25 bundles had been collected randomly and transported with minimum delay to the microbiology laboratory without cooling by the fridge [7].
Methods
• In this research 20 g of Cut Green Leaves and Parts of Stem (CGLPS) had been soaked in 800 ml of sterile distilled water and manual shaking for 3 minutes [8].
• The previously washed CGLPS had been soaked three times again with 800 ml of sterile distilled water and in each time the same method had been repeated.
0.1 ml of the last wash water had been inoculated onto nutrient agar media plates for determining the viable bacterial count CFU/ml of the sample. The CGLPS used after the third wash considered as a control for this study.
• After the 3rd wash, the 20 gm of CGLPS had been distributed into 4 portions equally (5 gm for each) put in sterile containers (beakers): ABC and D 200 ml of 0.1% potassium permanganate, 200 ml of 10% sodium chloride (table salt), 200 ml of 5% acetic acid (apple vinegar), and 200 ml of 2 ppm of sodium hypochloritehad been added to beaker ABC and D respectively. Each portion had been processed separately.
• CGLPS had been soaked in each solution for 10 min with shaking. The solutions had been decanted and sterile distilled water had been added to the samples and decanted to remove the traces of decontaminant solutions.
• The beakers had been refilled with distilled water and the contents had been shaken vigorously and the soaked water used to study bacterial counting.
• Of each portion 0.1 ml of the soaked water had been inoculated onto nutrient agar media plates for determining bacterial viability CFU/ml of the sample. All inoculated plates had been incubated in 37°C for 18 hrs-24 hrs.
All steps of experiments had been conducted at room temperature and use sterile distilled water.
Statistical analysis
Data obtained was analyzed by (ANOVA 1) method for multiple comparisons. Numeric data was expressed as (mean+SD), P-value (p<0.01) was considered significant when it was (P ≥ 0.05) and highly significant when it was (P ≥ 0.001). To generalize the mean count, 95% confidence interval had been used.
Results
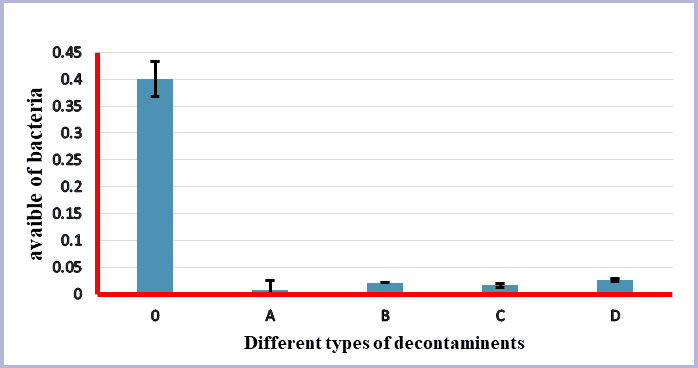
Figure 1 and Table 1 showing general description for the viable count of bacteria isolated from control and samples treated with different types of decontaminants.
| Decontaminants | N | Mean ± Std. Deviation | Std. Error | 95% Confidence interval for mean | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||||
| 0 | 25 | 0.4000±0.05292 | 0.03055 | 0.2686 | 0.5314 | 0.36 | 0.46 |
| A | 25 | 0.0071±0.00021 | 0.00012 | 0.0065 | 0.0076 | 0.01 | 0.01 |
| B | 25 | 0.0204±0.00113 | 0.00065 | 0.0176 | 0.0232 | 0.02 | 0.02 |
| C | 25 | 0.0169±0.00166 | 0.00096 | 0.0128 | 0.021 | 0.02 | 0.02 |
| D | 25 | 0.0255±0.00130 | 0.00075 | 0.0223 | 0.0287 | 0.02 | 0.03 |
Table 1. General description of the viable bacterial counts.
Table 2 shows the bacterial viable counts for portions of treatment in compare with control group.
| LSD (I) Group | Mean difference (I-J) | Std. Error | Sig. | 95% Confidence interval | ||
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| 0 | A | 0.39293* | 0.1934 | 0.000 | 0.3498 | 0.4360 |
| B | 0.37960* | 0.1934 | 0.000 | 0.3365 | 0.4227 | |
| C | 0.38310* | 0.1934 | 0.000 | 0.3400 | 0.4262 | |
| D | 0.37450* | 0.1934 | 0.000 | 0.3314 | 0.4176 | |
Table 2. LSD test to show the differences between control group and different treatment groups. *: The mean difference is significant at the 0.05 level.
Table 3 shows the differences in viability among different portions of treatment.
| LSD (I) Group | Mean difference (I-J) | Std. Error | Sig. | 95% Confidence interval | ||
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| 0 | A | 0.39293* | 0.1934 | 0.000 | 0.3498 | 0.4360 |
| B | 0.37960* | 0.1934 | 0.000 | 0.3365 | 0.4227 | |
| C | 0.38310* | 0.1934 | 0.000 | 0.3400 | 0.4262 | |
| D | 0.37450* | 0.1934 | 0.000 | 0.3314 | 0.4176 | |
| A | 0 | -0.39293-* | 0.1934 | 0.000 | -0.4360 | -0.3498 |
| B | -0.01333 | 0.1934 | 0.506 | -0.0564 | 0.0298 | |
| C | -0.00983 | 0.1934 | 0.622 | -0.0529 | 0.0333 | |
| D | -0.01843 | 0.1934 | 0.363 | -0.0615 | 0.0247 | |
| B | 0 | -0.3796-* | 0.1934 | 0.000 | -0.4227 | -0.3365 |
| A | 0.01333 | 0.1934 | 0.506 | -0.0298 | 0.0564 | |
| C | 0.0035 | 0.1934 | 0.860 | -0.0396 | 0.0466 | |
| D | -0.00510. | 0.1934 | 0.797 | -0.0482 | 0.038 | |
| C | 0 | -0.38310-* | 0.1934 | 0.000 | -0.4262 | -0.3400 |
| A | 0.00983 | 0.1934 | 0.622 | -0.0333 | 0.0529 | |
| B | -0.0035 | 0.1934 | 0.860 | -0.0466 | 0.0396 | |
| D | -0.00860 | 0.1934 | 0.666 | -0.0517 | 0.0345 | |
| D | 0 | -0.37450-* | 0.1934 | 0.000 | -0.4176 | -0.3314 |
| A | 0.01843 | 0.1934 | 0.363 | -0.0247 | 0.0615 | |
| B | 0.0051 | 0.1934 | 0.797 | -0.0380 | 0.0482 | |
| C | 0.0086 | 0.1934 | 0.666 | -0.0345 | 0.0517 | |
Table 3. One way a nova (LSD) showing the differences in viability among different groups of treatment. *: The mean difference is significant at the 0.05 level.
Discussion
The risk of human infections with different types of diseases can be reduced by removing or killing pathogens by washing and treating with decontaminants, although this could not kill all kinds of bacteria, but it can reduce their numbers in the most possible way. These treatments cannot completely eliminate bacteria for several reasons, including the formation of biofilm, or may be bacteria become internalized inside plant tissues [9].
In this research, samples had been used directly to prevent refrigerating that influenced microbial growth and may reduce the real bacterial count [7,10]. Samples had been washed three times before starting treating with decontaminants firstly, because many references mention the importance of washing with water to reduce microbial content [7,11-13], and secondly, because the presence of organic materials and soil particles reduces the efficiency of decontaminants used [12,14]. Washed samples had been considered as control for statistical analysis of significances; it is clear from Figure 1 and Table 1 the reduction of the viable bacterial count after usage of different types of decontaminants [15].
All types of decontaminants were statistically significant and have a good action against bacterial viability, potassium permanganate in portion A was the most effective decontaminant used in this study this appeared in Table 2 in a comparison of different portions results with the control. Potassium permanganate kills bacteria especially coliforms it acts as an astringent, which is a drying agent [16,17]. Salt and vinegar were also effective in portion B and C, but not as potassium permanganate. Using sodium hypochlorite at higher concentrations may give much better results, but higher concentrations of this substance are prohibited because they have a toxic effect on the human body cells [14,18-20].
Conclusion
From the above we conclude that the use of decontaminants of different types and at acceptable concentrations for human consumption after washing green vegetables with water has a very positive effect by reducing the number of bacteria and thus preventing their harmful effect. The most effective substance is 0.1% potassium permanganate, second 5% vinegar, third 10% table salt and fourth 2 ppm sodium hypochlorite.
Limitations
• This study had been done only in vitro cultivable bacteria. Bacterial isolates have not been further characterized for genera and species, properties, or drug resistance.
• Effects of different substances had been tested for a single concentration for each one and the same time of soaking. • Table salt used in this research consist of 99.9% Sodium Chloride and 0.008% magnesium carbonate+potassium iodide.
References
- Larsson SC, Bergkvist L, Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: A prospective study. Cancer Epidemiol Biomarkers Prev 2006; 15(10): 1998-2001.
- http://www.fao.org/fao-who-codexalimentarius/en/
- Denis N, Zhang H, Leroux A, et al. Prevalence and trends of bacterial contamination in fresh fruits and vegetables sold at retail in Canada. Food Control 2016; 67: 225-34.
- http://www.fao.org/3/y4893e/y4893e.pdf
- Feliziani E, Lichter A, Smilanick JL, et al. Disinfecting agents for controlling fruit and vegetable diseases after harvest. Post Harvest Biol Technol 2016; 122: 53-69.
- Wang J, Yu Y, Dong Y. Disinfection of ready-to-eat lettuce using polyhexamethylene guanidine hydrochloride. Microorganisms 2020; 8(2): 272.
- Phil N, Mcinerney S, Prendergast M. Growth dynamics of indigenous microbial populations on vegetables after decontamination and during refrigerated storage. J Food Process Preserv 2004; 28(6): 442-59.
- Subramanya SH. Potassium permanganate cleansing is an effective sanitary method for the reduction of bacterial bioload on raw Coriandrum sativum. BMC Res Notes 2018; 11(124): 1-5.
- Arogbodo JO, Faluyi OB, Igbe FO. In vitro antimicrobial activity of ethanolic leaf extracts of hibiscus asper hook. F. and hibiscus sabdariffaL. on some pathogenic bacteria. J Sci Res Med Biol Sci 2021; 2(3): 1-12.
- Banach JL, Sampers I, Haute SV, et al. Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int J Environ Res Public Health 2015; 12(8): 8658-77.
- Ríos-Castillo AG, Ripolles-Avila C, Rodríguez-Jerez JJ. Evaluation of bacterial population using multiple sampling methods and the identification of bacteria detected on supermarket food contact surfaces. Food Control 2020; 119: 107471.
- Barrera MJ, Blenkinsop R, Warriner K. The effect of different processing parameters on the efficacy of commercial post-harvest washing of minimally processed spinach and shredded lettuce. Food Control 2012; 25(2): 745-51.
- Kambhampati V, Singh SS, Mohanty S. A review on postharvest management and advances in the minimal processing of fresh-cut fruits and vegetables. J Microbiol Biotechnol Food Sci 2019; 8(5): 1178-87.
- Turki HK, Ali ZM, Hussein SA. Effect of washing and prevalence of bacteria in leafy vegetable foods collected from Iraqi markets. Plant Arch 2020; 20(2): 6925-8.
- Shoukry LR, Mohamed AN, Sharaf AEA, et al. Diagnostic markers for early detection of neonatal sepsis. J Scientific Res Med Biol Sci 2021; 2(3): 13-26.
- Rutala WA, Weber DJ, Healthcare Infection Control Practices Advisory Committee (HICPAC). Guideline for disinfection and sterilization in healthcare facilities, 2008. Centers for Disease control and Prevention. 2019; pp:1-163.
- Sood B, Sahota PP, Kaur K, et al. Efficacy of decontaminating agents for raw vegetable consumption and sensory screening. J Pure Appl Microbiol 2017; 11(3): 1497-1507.
- Dadhich AS, Khan HR. Elimination of microbes from different drinking water sources of Visakhapatnam using potassium permanganate: Dose based disinfection approach. Int Lett Nat Sci 2014; 11(1): 11-18.
- Amoah P, Drechsel P, Abaidoo RC, et al. Effectiveness of common and improved sanitary washing methods in selected cities of West Africa for the reduction of coliform bacteria and helminth eggs on vegetables. Trop Med Int Health 2007; 12(Suppl 2): 40-50.
- Haute VS, Sampers I, Holvoet K, et al. Physicochemical quality and chemical safety of chlorine as a reconditioning agent and wash water disinfectant for fresh-cut lettuce washing. Appl Environ Microbiol 2013; 79(9): 2850-61.