Research Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2018) Volume 8, Issue 65
The Effect of Alcoholic Extract of Ginger on Apoptosis and Inflammation of Germ Cells in Streptozotocin-Induced Diabetic Rats
Mohammad MT1, Mahdi SK2, Zahra T2, Ahmad S2*, Mohammad KA3, Akram M4, Hamid RJ4
1Department of Anatomy, Faculty of Medicine, Social Determinants of Health Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
2Department of Anatomy, Faculty of Medicine, Immunology of Infectious Diseases Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
3Department of Immunology, Faculty of Medicine, Immunology of Infectious Diseases Research Center, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
4Department of Anatomy, Faculty of Medicine, Rafsanjan University of Medical Sciences, Rafsanjan, Iran
- *Corresponding Author:
- Dr. Ahmad Shabanizadeh
Department of Anatomy, Faculty of Medicine
Immunology of Infectious Diseases Research Center
Rafsanjan University of Medical Sciences
Rafsanjan, Iran
Tel: +98916273475
E-mail: shabani54@yahoo.com
Accepted date: June 11, 2018
Keywords
Ginger, Apoptosis, Germ cells, Testis, Diabetes.
Introduction
The number of diabetes cases in the world is growing increasingly. It is estimated that the number of diabetic patients in 2025 will exceed 300 million [1]. Sexual activity for both sexes (male and female) will be affected by diabetes. Unfortunately, many patients may be at reproductive age. It is reported that the two types of diabetes cause a range of female sexual dysfunctions such as: loss of libido, vaginal dryness and discomfort [2], higher prevalence of reduced vasocongestion and reduced vaginal lubrication to erotic stimuli [3], reduced sensitivity of the clitoris [4] and so on. In the male, a wide range of abnormalities and problems related to reproductive system have been reported in both diabetic patients and diabetic animal models. Some of these changes such as low plasma levels of gonadotrophins and testosterone were similar in both animal and human [5].
Abnormal morphology of sperm, loss of libido, and erectile dysfunction have been reported in diabetic patients, but atrophy of sex organ, changes in histoarchitecture of ventral prostate, lower sperm count and motility, reduced seminal fluid volume, are the main reported disorders in animal models [6,7]. Apoptosis in testis of diabetic men has been observed and infertility in diabetic men may be associated with degeneration of sperm germ cells, not only in spermatogonia but also in spermatocytes and spermatids [8]. Although some researchers believe that more than apoptosis other factors such as decrease in the numbers of luteinizing hormones receptors in leydig cells, low testosterone production, and microangiopathy with thickening of the testicular microvascular basement membranes are involved in male diabetic infertility [9]. Why does apoptosis increase in diabetes? It seems that the formation of oxidative stress is increased by hyperglycemia in diabetes and as a result, the antioxidant capacity changes play a significant role in increased apoptosis in testis of diabetic men [9].
Additionally, it has been reported that toll-like receptors (TLRs) and TRPV1 are the main receptors and canal, respectively, which participate in the induction of inflammation in the tissues which can lead to enhanced apoptosis [10,11]. Accordingly, it seems that the complications of diabetes in the testis can be associated with the expression of molecules. Therefore, a suitable therapeutic strategy can be considered for treatment of diabetes to reduce the risk of testis complication. Chemical or synthetic drugs have many side effects, so in many countries, traditional medicine, especially herbal medicines have been used to treat some of the complications of diabetes.
Zingiber Officinale Rosce is the scientific name of Ginger plant. Ginger is extensively used as a spice and food preservative in Southeast Asia, India and China. For many years, in countries like China and India ginger has been used to treat diseases such as nausea, stomach pain, gingivitis, tooth pain, digestive disorders, arthritis, respiratory disorders, atherosclerosis, migraine, depression, ulcers, high cholesterol, etc [12]. Thus, it seems that the herbal drug can be considered as an important candidate for treatment of diabetes to avoid testis complications. Accordingly, this study was aimed to evaluate the likely therapeutic effects of alcoholic extract of ginger on apoptosis of germ cells of in streptozotocin-induced diabetic rats as well as expression of apoptosis related molecules including Bax, Bcl-2, CyclinD1, TRPV1, TLR2 and TLR4.
Methods
Animals and experimental protocol
In this experimental study, male wistar rats at an average weight of 200-230 g were maintained in the animal house of the Faculty of Medicine, Rafsanjan University of Iran. All animals were maintained under controlled conditions of temperature (23 ± 2 °C) and light (12 h light/dark cycles). Animals received standard pellet food and water ad libitum. The experimental protocol was approved by the University Animal Care Committee. After weighing, rats were divided to five groups (n=12) as follows: non-treatment normal group (group 1), non- treatment diabetic group (sham group, group 2), ginger treatment diabetic group (group 3), insulin treatment diabetic group (group 4) and ginger-insulin treatment diabetic group (group 5). The first and second groups received saline only (equal volume). Diabetes was induced by injection of 60 mg/kg streptozotocin in the four remaining diabetic groups. After three days, blood was collected from the tip of the tail vein and Blood glucose levels were measured with a glucometer (Accucheck Glucose Meter, Roche, Switzerland). Animals with blood glucose levels of 220 mg/dl and above were taken into the study [13].
In addition, diabetic animals had symptoms such as polydipsia, polyuria, overeating. In addition to ginger, animals in the last group received insulin 4-6 units/kg each day. Ginger extract was injected (200 mg/kg every other day) in the two last groups. Ginger injections were done for 56 consecutive days. Twenty-four hours after the last injection, animals were anesthetized with ether and after dissecting the scrotum and other covers around the testis, both testis with tunica albuginea were removed. Testicles were used to evaluate the apoptosis by two different methods, Right testis for TUNEL method and left testis for real time-PCR.
Preparation of ginger extract
Ginger rhizome was prepared from Esfahan Plant Herbarium Company. Rhizome was powdered and equal volume of alcohol 50% was added to ginger powder. The mixture was kept at 70-80°C for 15 h in water bath. The suspension was centrifuged for 10 minutes at 2500 g. In order for all of the alcohol to evaporate, the supernatant was held in a water bath at 70-80°C. Finally, in order to obtain ginger stock solution, the appropriate amount of the originated extract was calculated and dissolved into the proportional volume of PBS. The stock solution was kept in a refrigerator until used [12].
In situ germ cell apoptosis detection
Right testicles were used to evaluate apoptosis by TUNEL method. The right removed testicles were put in bouin solution (as fixative) for 24 h. For deeper penetration of the fixative solution to the deep part of the testicles, each pole of testis was penetrated with a needle. To eliminate the yellow colour of picric acid from tissue, firstly, testicles were transferred to 70% ethanol for 2 days and alcohol was replaced 3 times each day. Secondly, tissues were transferred several times to a saturated solution of ethylic alcohol 70% and lithium carbonate. Thirdly, various stages of preparing tissue to sectioning such as dehydration, infiltration, embedding were done.
Finally, sections with a thickness of 7 μm were cut using a microtome. In situ cell death detection kit was purchased from Roche (Germany). Briefly, Germ cell apoptosis was examined as following: Seven micrometer thick sections were deparaffinized, rehydrated, washed and incubated with proteinase K. After adding H2O2 to quench peroxidase activity, again sections were incubated, firstly with the TUNEL reaction mixture [terminal deoxynucleotidyl transferase plus the nucleotide mixture in reaction buffer], and secondly with converter-AP [containing antifluorescein antibody (Fab fragment from sheep) conjugated with Alkaline Phosphatase (AP)], and thirdly with substrate solution [containing 7 mg diaminobenzidine (DAB) (Sigma) and 3 μl H2O2 in 10 ml of 50 mM Tris-HCl, pH 7.4]. Incubations were performed in moist chamber. Finally, samples were counterstained with Hematoxylin. Positive control sections were incubated with micrococcal nuclease or recombinant DNase1 to induce DNA strand breaks prior to labelling. Negative control sections were processed with label solution (without terminal transferase) [14,15].
Real time PCR
Left testicles were used to study apoptotic gene expression (Bax, Bcl-2, CyclinD1, TRPV1, TLR2 and TLR4) by real time PCR. The removed testicles were kept in sterile tubes containing ACSF at -80 until further processing. Then, testicles were homogenized in sterile condition and RNA was extracted to make cDNA. The β-actin gene was used as an internal control. The primers used have been shown in Table 1.
Total RNA was extracted from testis using Trizol Reagent (Invitrogen, Carlsbad, CA). The purity of extracted RNA was determined by electrophoresis on an ethidium bromide pre pretreated agarose gel along with measuring absorption by spectrophotometer and calculation of 260/280 ratio. The RNA was converted to cDNA using a cDNA synthesis kit (Bionner, Korea) with both oligo (dT) and random hexamer primers. The process of reverse transcription was performed by the following protocol: 70°C for 10 min (without reverse transcription enzyme), 20°C for 1 min (cooling step), addition of reverse transcription enzyme, 42°C for 60 min, and the protocol was completed by final step at 95°C for 10 min to terminate the activation of the reverse transcription enzyme.
Real-time PCR was performed using a SYBR green master mix (Bionner, Korea), combined with 200 mg of template cDNA with the appropriate primers (Table 1) in a Bio-Rad CFX96 system (Bio-Rad Company, USA) using the following program: 1 cycle of 95°C for 15 min, 40 cycles of 95°C for 30 s and 60°C for 30 s and finally, 72°C for 30 s. Primers were approved by Blast after design and synthesized by the Bionner Company (Korea).
| Bax (F) | GATGGCAACTTCAACTGGGG |
| Bax (R) | AGCCACCCTGGTCTTGGAT |
| Bcl-2 (F) | TGGCCTTCTTTGAGTTCGGT |
| Bcl-2 (R) | AGTTCCACAAAGGCATCCCAG |
| Cyclin D1 (F) | CAAGTGTGACCCGGACTGC |
| Cyclin D1 (R) | CACATCTCGCACGTCGGT |
| TRPV1 (F) | GACATGCCACCCAGCAGG |
| TRPV1 (R) | TCAATTCCCACACACCTCCC |
| TLR2 (F) | CTGATGGAGGTGGAGTTTGA |
| TLR (R) | TCCGTATTGTTACCGTTTCTA |
| TLR4 (F) | GAATTGTATCGCCTTCTTAG |
| TLR4 (R) | TGTGAGGTCGTTGAGGTTAG |
| Betta-Actin (F) | CTGTGCTGCTCACCGAGG |
| Betta-Actin (R) | CGGAGTCCATCACAATGCCT |
Table 1: The designed primers for the gene expression of Bax, Bcl-2, Cyclin D1, TRPV1, TLR2 and TLR4 in testis.
Real-time PCR was carried out in triplicate and the ß-actin was applied as a housekeeping gene for normalization of the amplified signals of the target genes. The sequences of the used primers are shown in Table 1. The quantity of cytokines mRNA in the testis was expressed as units relative to the amount of ß-actin mRNA. The dissociation stages, melting curves and quantitative analyses of the data were performed using CFX manager software version 1.1.308.111 (Bio-Rad, USA) [15-17].
Results
Our results show that the body weight decreased significantly in all diabetic groups at the end of study. In ginger insulin diabetic group, the weight of testis was the same as normal group, but in comparison to non-treatment diabetic group was significantly different (p ≤ 0.001) (Table 2).
| Group | Animal weight before starting the experiments | animal weight at the end of experiment | testis weight | testis weight/body weight ratio |
|---|---|---|---|---|
| 1 | 215.5 ア 6.17 | 221.33 ア 8.18 | 1.24 ア 0.03 | 0.0056 ア 0.00029 |
| 2 | 213.42 ア 9.59 | 121.67 ア 8.28 | 0.72 ア 0.06 | 0.0061 ア 0.00072 |
| 3 | 215.33 ア 10.83 | 159.83 ア 7.07*** | 0.77 ア 0.09 | 0.0048 ア 0.00067*** |
| 4 | 217.25 ア 10.9 | 187.17 ア 4.41*** | 0.80 ア 0.09 | 0.0043 ア 0.00050*** |
| 5 | 214.67 ア 10.82 | 194.17 ア 3.74*** | 1.18 ア 0.05*** | 0.0062 ア 0.00050 |
Table 2: Body and testis weights of animals in different studied groups.
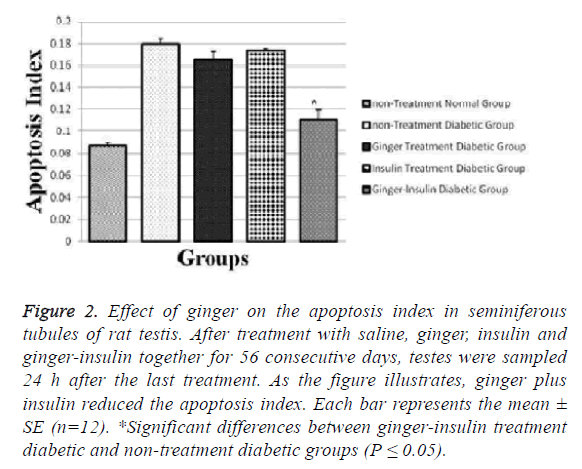
Mean amount of calculated apoptosis index in the gingerinsulin treatment diabetic and non-treatment diabetic groups was 0.111 ± 0.008 and 0.180 ± 0.004 respectively, showing decreased apoptosis in the ginger-insulin treatment diabetic group in comparison with sham group after injections (p ≤ 0.05).
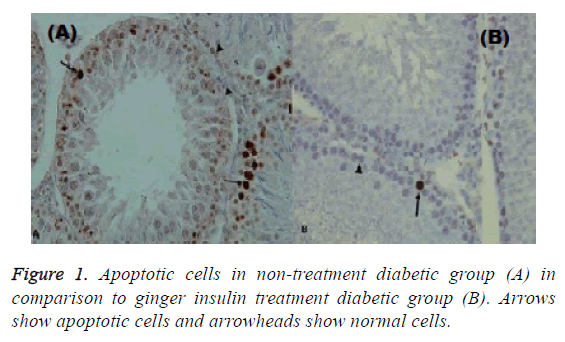
In this study, insulin is the mainstay of treatment, so if ginger is given alone it cannot be treated but if used with insulin it will be effective in reducing apoptosis and inflammation. Though it reduces apoptosis and inflammation but it is not significant. Apoptosis indices in non-treatment normal, ginger treatment and insulin treatment groups were 0.087 ± 0.003, 0.165 ± 0.007 and 0.0173 ± 0.002, respectively (Figures 1 and 2).
Figure 2: Effect of ginger on the apoptosis index in seminiferous tubules of rat testis. After treatment with saline, ginger, insulin and ginger-insulin together for 56 consecutive days, testes were sampled 24 h after the last treatment. As the figure illustrates, ginger plus insulin reduced the apoptosis index. Each bar represents the mean ± SE (n=12). *Significant differences between ginger-insulin treatment diabetic and non-treatment diabetic groups (P ≤ 0.05).
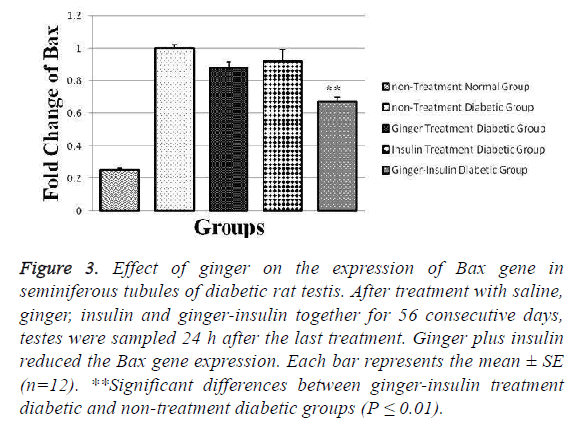
Our gene expression results showed that in the non-treatment diabetic group, the Bax gene was expressed less than other diabetic groups and there was a significant difference between mentioned group and ginger-insulin treatment diabetic group (p ≤ 0.01). As predicted, in the non-treatment normal group the expression of Bax gene (0.025 ± 0.010) was lower than other groups (Figure 3).
Figure 3: Effect of ginger on the expression of Bax gene in seminiferous tubules of diabetic rat testis. After treatment with saline, ginger, insulin and ginger-insulin together for 56 consecutive days, testes were sampled 24 h after the last treatment. Ginger plus insulin reduced the Bax gene expression. Each bar represents the mean ± SE (n=12). **Significant differences between ginger-insulin treatment diabetic and non-treatment diabetic groups (P ≤ 0.01).
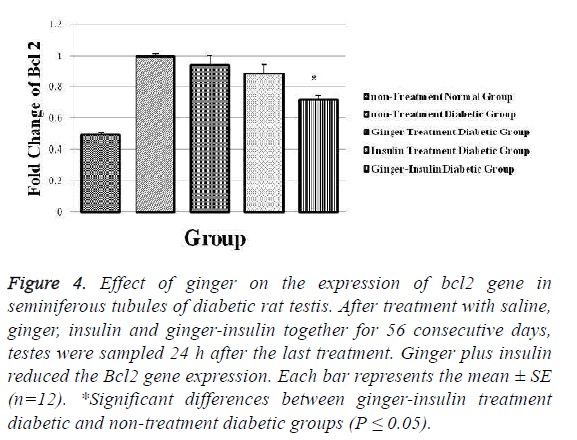
The expression of Bcl2 mRNA was 1 ± 0.023 and 0.72 ± 0.032 for non-treatment diabetic and ginger-insulin treatment diabetic groups respectively, so the Bcl2 expression decreased significantly in ginger-insulin treatment diabetic group in comparison to non-treatment diabetic group (p ≤ 0.05) (Figure 4).
Figure 4: Effect of ginger on the expression of bcl2 gene in seminiferous tubules of diabetic rat testis. After treatment with saline, ginger, insulin and ginger-insulin together for 56 consecutive days, testes were sampled 24 h after the last treatment. Ginger plus insulin reduced the Bcl2 gene expression. Each bar represents the mean ± SE (n=12). *Significant differences between ginger-insulin treatment diabetic and non-treatment diabetic groups (P ≤ 0.05).
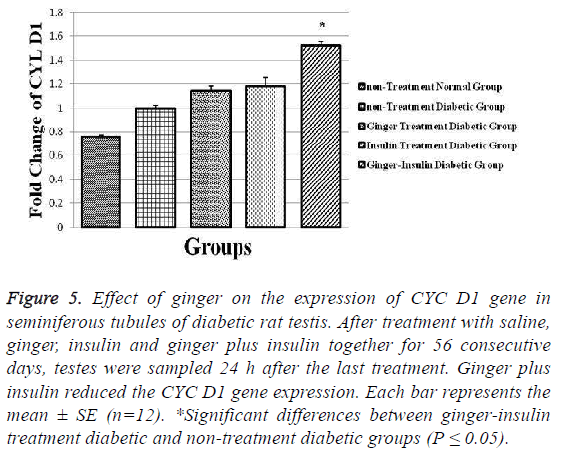
Unlike previous genes, the expression of CYC D1 gene increased significantly in ginger insulin treatment diabetic group in comparison to non-treatment diabetic group (p ≤ 0.05). The highest and lowest values obtained for this parameter were 0.76 ± 0.023 and 1.52 ± 0.042 respectively in non-treatment normal and ginger insulin treatment diabetic groups (Figure 5).
Figure 5: Effect of ginger on the expression of CYC D1 gene in seminiferous tubules of diabetic rat testis. After treatment with saline, ginger, insulin and ginger plus insulin together for 56 consecutive days, testes were sampled 24 h after the last treatment. Ginger plus insulin reduced the CYC D1 gene expression. Each bar represents the mean ± SE (n=12). *Significant differences between ginger-insulin treatment diabetic and non-treatment diabetic groups (P ≤ 0.05).
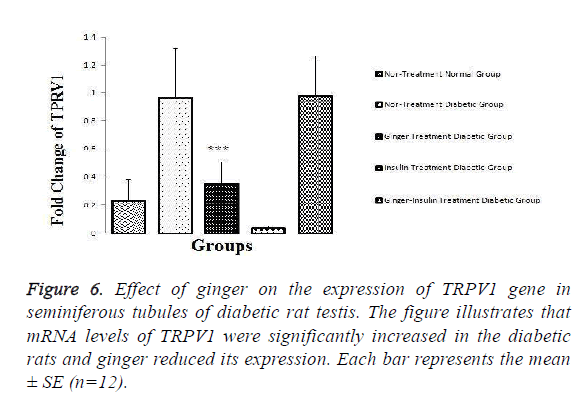
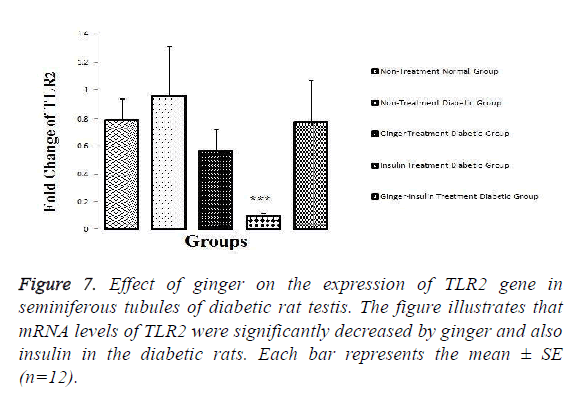
The results showed that mRNA levels of TRPV1 (P=0.026), TLR2 (P=0.026) and TLR4 (P=0.05) were significantly increased in the testis of the diabetic in comparison to normal healthy rats. The results also revealed that the expression of TRPV1 (P=0.006) and TLR2 (P=0.006) was significantly decreased in group 3 and 4 when compared with non-treated diabetic controls (Figures 6 and 7).
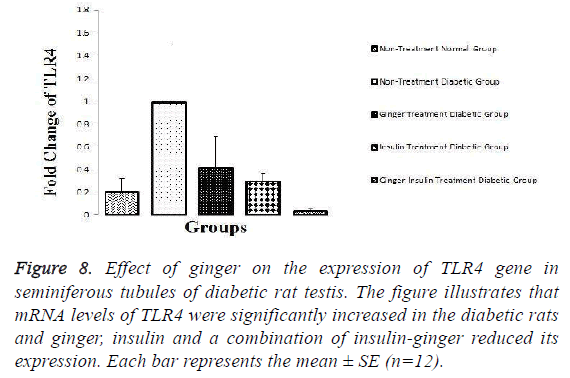
While only ginger in combination with insulin (group 5) can decrease mRNA levels of TLR4 (Figure 8).
Figure 8: Effect of ginger on the expression of TLR4 gene in seminiferous tubules of diabetic rat testis. The figure illustrates that mRNA levels of TLR4 were significantly increased in the diabetic rats and ginger, insulin and a combination of insulin-ginger reduced its expression. Each bar represents the mean ± SE (n=12).
As it is illustrated in the Figures 6-8, expression levels of TRPV1, TLR2 and TLR4 were not altered among other groups.
Discussion
Our results firstly, showed that the ginger extract could reduce, albeit at low level, apoptosis of sperm germ cells in diabetic groups, especially when ginger extract is used in combination with insulin. Secondly, increase of the CYCD1 gene expression in our results may represent a positive effect of extract on cell proliferation. According to the result of previous studies the ginger may be able to reduce germ cells apoptosis via decrease in blood sugar. The most important factor in herbal drugs in reduction of blood sugar is antioxidant compounds, but control of blood sugar alone is not able to prevent the diabetes complications [18]. With regard to the role of free radicals in diabetes, one of the areas of research in treatment of the disease is reduction of these radicals. In this context, the effect of antioxidants in preventing damages resulting from free radicals has been studies in diabetic diseases [3]. Since the oxidative stress results from an imbalance between the production of free radicals and reactive oxygen species, antioxidant defence mechanism must be able to reduce the harmful effects of these aggressive agents [15].
Therefore, an ideal drug for treatment of the diabetes must not only reduce the blood sugar level, but it should also have some antioxidant properties. To deal with free radicals and reactive oxygen species, chemical drugs which lower blood sugar should be able to control tissue damages in this disease, but they could also cause numerous side effects. Diabetes treatment without any side effects is still not completely possible and remains a challenge for the health system. Due to side effects of chemical synthetic drugs, researcher's attention has recently focused on the herbal drugs [19]. Diabetic diseases tend to use natural products, because the common hypoglycemic drugs have undesirable side effects. The potential role of medicinal plants as hypoglycemic agents has been seen in many botanical studies and the use of traditional medicinal plants has been reported in different cultures. In addition to being a good source of natural antioxidants, herbal medicines impose less financial pressure on the family economics of patients [20].
In Chinese and Indian ancient medicine, ginger is used to treat different diseases such as nausea, stomach and tooth pain, inflammation of gums, digestive and respiratory disorders, arthritis, atherosclerosis, migraine, depression, ulcer. Studies have shown that in addition to antioxidant and antiinflammatory properties, the ginger reduced the blood sugar with the antagonistic activity against serotonin receptors. It is known that treatment with antioxidants may improve glucose transport and its tolerance in patients with diabetes type 2 and animals with insulin resistance. Also, ginger with antioxidants including gingerols and shograns prevents the active metabolites of free radicals and eliminates these free radicals. Additionally, the results revealed that expression levels of TRPV1, TLR2 and TLR4 were significantly increased in group 2 (non-treated diabetic animals) in comparison to group 1 (healthy animals). Based on the fact that the molecules are potentially involved in the induction of inflammation, it appears that diabetes can enhance inflammation in the testis via several mechanisms including TLRs and also TRPV1 pathways. Interestingly, the results demonstrated that ginger and insulin were able to decrease expression levels of TRPV1 and TLR2. Based on the fact that TRPV1 is an important canal to induce inflammation and also TLR2 is a molecule with dual functions such as induction of inflammation, it may be concluded that ginger and insulin play critical roles in reduction of inflammation and can be used for inhibition of diabetes complication in the testis.
Additionally, due to the fact that, TLR4 uses both MYD88 and TRIF dependent pathways and based on the results which showed that only ginger in combination with insulin, but not in separate format, is able to decrease expression of TLR4, it appears that insulin and ginger are unable to decrease TLR4- related inflammation in separate administration. To the best of our knowledge, there are no related investigations regarding the roles of ginger and its combination with insulin on the expression of TLR2, but some investigations have evaluated the effects of ginger on the expression of TLR4 and TRPV1 in other animal models rather than diabetic animal model. For instance, a study revealed that ginger has an interaction with TRPV1 to decrease expression of IFN-γ, as inflammatory cytokines, by human T lymphocytes [21]. Another study reported that ginger can activate TRPV1 channels and consequently induce Ca² signals in the beta-cell which leads to sensitizing the beta-cells to glucose [22]. The effects of ginger on the expression and functions of TRPV1 have also been documented by several investigations [23,24]. Additionally, Ahn et al. showed that ginger inhibits homodimerization of TLR4 which suppress its functions [25]. Another study revealed that ginger suppresses expression of inflammatory genes in a NF-κB dependent manner [26]. NF-κB is a main transcription factor down-stream of TLR2 and 4 and participates in the expression of the TLRs [27-29].
Conclusion
Based on the results, it seems that ginger can be used as an important drug to decrease diabetes related inflammation in the testis.
Funding
This article has been supported by Rafsanjan University of Medical Sciences.
Acknowledgements
The current article is supported by a grant from Rafsanjan University of Medical Sciences.
References
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414-1431.
- Kim NN, Stankovic M, Cushman TT, Goldstein I, Munarriz R, Traish AM. Streptozotocin-induced diabetes in the rat is associated with changes in vaginal hemodynamics, morphology and biochemical markers. BMC Physiol. 2006;6:4.
- Mallick C, Mandal S, Barik B, Bhattacharya A, Ghosh D. Protection of testicular dysfunctions by MTEC, a formulated herbal drug, in streptozotocin induced diabetic rat. Biol Pharm Bull. 2007;30:84-90.
- Elyasi F, Kashi Z, Tasfieh B, Bahar A, Khademloo M. Sexual dysfunction in women with type 2 diabetes mellitus. Iran J Med Sci. 2015;40:206-213.
- Beatrice AM, Dutta D, Kumar M, Kumbenahalli Siddegowda S, Sinha A, Ray S, Chowdhury S. Testosterone levels and type 2 diabetes in men: current knowledge and clinical implications. Diabetes Metab Syndr Obes. 2014;7:481-486.
- Santos SA, Rinaldi JC, Martins AE, Camargo AC, Leonelli C, Delella FK, Felisbino SL, Justulin LA, Jr., Impact of gestational diabetes and lactational insulin replacement on structure and secretory function of offspring rat ventral prostate. Gen Comp Endocrinol. 2014;206:60-71.
- Giribabu N, Kumar KE, Rekha SS, Muniandy S, Salleh N. Chlorophytum borivilianum (Safed Musli) root extract prevents impairment in characteristics and elevation of oxidative stress in sperm of streptozotocin-induced adult male diabetic Wistar rats. BMC Complement Altern Med. 2014;14:291.
- Kilarkaje N, Al-Bader MM. Diabetes-induced oxidative dna damage alters p53-p21CIP1/Waf1 signaling in the rat testis. Reprod Sci. 2015;22:102-112.
- Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive
- function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008;4:46-54.
- Zare-Bidaki M, Tsukiyama-Kohara K, Arababadi MK. Toll-like receptor 4 and hepatitis B infection: molecular mechanisms and pathogenesis. Viral Immunol. 2014;27:321-326.
- Hakimizadeh E, Arababadi MK, Shamsizadeh A, Allahtavakoli M, Rezvani ME, Roohbakhsh A. Morphine Reduces Expression of TRPV1 Receptors in the Amygdala but not in the Hippocampus of Male Rats. Iran J Bas Med Sci. 2014;39:261-267.
- Ajith TA, Nivitha V, Usha S. Zingiber officinale Roscoe alone and in combination with alpha-tocopherol protect the kidney against cisplatin-induced acute renal failure. Food Chem Toxicol. 2007;45:921-927.
- Jafari Anarkooli I, Barzegar Ganji H, Pourheidar M. The Protective Effects of Insulin and Natural Honey against Hippocampal Cell Death in Streptozotocin-Induced Diabetic Rats. J Diabetes Res. 2014.
- Alavi SH, Taghavi MM, Moallem SA. Evaluation of effects of methamphetamine repeated dosing on proliferation and apoptosis of rat germ cells. Syst Biol Reprod Med. 2008;54:85-91.
- Li M, Liu Z, Zhuan L, Wang T, Guo S, Wang S, Liu J, Ye Z. Effects of apocynin on oxidative stress and expression of apoptosis-related genes in testes of diabetic rats. Mol Med Rep. 2013;7:47-52.
- Jiang X, Zhang C, Xin Y, Huang Z, Tan Y, Huang Y, Wang Y, Feng W, Li X, Li W, Qu Y, Cai L. Protective effect of FGF21 on type 1 diabetes-induced testicular apoptotic cell death probably via both mitochondrial- and endoplasmic reticulum stress-dependent pathways in the mouse model. Toxicol Lett. 2013;219:65-76.
- Jafarzadeh A, Azizi SV, Nemati M, Khoramdel-Azad H, Shamsizadeh A, Ayoobi F, Taghipour Z, Hassan ZM. Ginger Extract Reduces the Expression of IL-17 and IL-23 in the Sera and Central Nervous System of EAE Mice. Iran J Immunol. 2015;12:288-301.
- Amin A, Hamza AA, Kambal A, Daoud S. Herbal extracts counteract cisplatin-mediated cell death in rat testis. Asian J Androl. 2008;10:291-297.
- Khaki A, Khaki AA, Hajhosseini L, Golzar FS, Ainehchi N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr J Tradit Complement Altern Med. 2014;11:1-8.
- Shalaby MA, Hamowieh AR. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol. 2010;48:2920-2924.
- Schoenknecht C, Andersen G, Schmidts I, Schieberle P. Quantitation of Gingerols in Human Plasma by Newly Developed Stable Isotope Dilution Assays and Assessment of Their Immunomodulatory Potential. J Agricultural Food Chem. 2016;64:2269-2279.
- Rebellato P, Islam MS. [6]-shogaol induces Ca2+ signals by activating the TRPV1 channels in the rat insulinoma INS-1E cells. J Pancreas. 2014;15:33-37.
- Okumi H, Tashima K, Matsumoto K, Namiki T, Terasawa K, Horie S. Dietary agonists of TRPV1 inhibit gastric acid secretion in mice. Planta Med. 2012;78:1801-1806.
- Khan K, Singh A, Mittal M, Sharan K, Singh N, Dixit P, Sanyal S, Maurya R, Chattopadhyay N. [6]-Gingerol induces bone loss in ovary intact adult mice and augments osteoclast function via the transient receptor potential vanilloid 1 channel. Mol Nutr Food Res. 2012;56:1860-1873.
- Ahn SI, Lee JK, Youn HS. Inhibition of homodimerization of toll-like receptor 4 by 6-shogaol. Mol Cells. 2009;27:211-215.
- Lee HY, Park SH, Lee M, Kim HJ, Ryu SY, Kim ND, Hwang BY, Hong JT, Han SB, Kim Y. 1-Dehydro-[10]-gingerdione from ginger inhibits IKKbeta activity for NF-kappaB activation and suppresses NF-kappaB-regulated expression of inflammatory genes. Br J Pharmacol. 2012;167:128-140.
- Bagheri V, Askari A, Arababadi MK, Kennedy D. Can Toll-Like Receptor (TLR) 2 be considered as a new target for immunotherapy against hepatitis B infection? Hum Immun. 2014;75:549-554.
- Radman M, Golshiri A, Shamsizadeh A, Zainodini N, Bagheri V, Arababadi MK, Kennedy D. Toll-like receptor 4 plays significant roles during allergic rhinitis. Allergol Immunopathol (Madr). 2015;43:416-420.
DOI: 10.4066/2249-622X.65.18-644
Visit for more related articles at Asian Journal of Biomedical and Pharmaceutical SciencesAbstract
Background: Herbal drugs have been used to treat several diseases from liver and neural disorders to diabetes. Our study was aimed to find out the effects of alcoholic ginger extract on testis/body weight and apoptosis of sperm germ cells of chronic diabetic rat. Methods: In this experimental study, sixty male Wistar rats were randomly divided into 5 groups: nontreatment normal (group 1), non-treatment diabetic (group 2), ginger treatment diabetic (group 3), insulin treatment diabetic (group 4) and ginger plus insulin treatment diabetic (group 5). Ginger alcohol extract was injected intraperitoneally at the dose of 200 mg/kg for 6 weeks. TUNEL method was employed to evaluate the germ cells apoptosis. Expression of Bax, Bcl-2, CyclinD1, TRPV1, TLR2 and TLR4 was evaluated using Real-Time PCR technique. Results: Results revealed a significant decrease in the apoptosis index in the group 5 with respect to the group 2 (P<0.05). The expression of BAX, BCL2, TRPV1, TLR2 and TLR4 significantly decreased, while CYC D1 was increased in the group 5 in comparison to group 2. Conclusions: These results suggest ginger has a potentially beneficial role in reducing apoptosis and inflammation in testicular germ cells in chronic diabetic adult rats.
Keywords
Ginger, Apoptosis, Germ cells, Testis, Diabetes.
Introduction
The number of diabetes cases in the world is growing increasingly. It is estimated that the number of diabetic patients in 2025 will exceed 300 million [1]. Sexual activity for both sexes (male and female) will be affected by diabetes. Unfortunately, many patients may be at reproductive age. It is reported that the two types of diabetes cause a range of female sexual dysfunctions such as: loss of libido, vaginal dryness and discomfort [2], higher prevalence of reduced vasocongestion and reduced vaginal lubrication to erotic stimuli [3], reduced sensitivity of the clitoris [4] and so on. In the male, a wide range of abnormalities and problems related to reproductive system have been reported in both diabetic patients and diabetic animal models. Some of these changes such as low plasma levels of gonadotrophins and testosterone were similar in both animal and human [5].
Abnormal morphology of sperm, loss of libido, and erectile dysfunction have been reported in diabetic patients, but atrophy of sex organ, changes in histoarchitecture of ventral prostate, lower sperm count and motility, reduced seminal fluid volume, are the main reported disorders in animal models [6,7]. Apoptosis in testis of diabetic men has been observed and infertility in diabetic men may be associated with degeneration of sperm germ cells, not only in spermatogonia but also in spermatocytes and spermatids [8]. Although some researchers believe that more than apoptosis other factors such as decrease in the numbers of luteinizing hormones receptors in leydig cells, low testosterone production, and microangiopathy with thickening of the testicular microvascular basement membranes are involved in male diabetic infertility [9]. Why does apoptosis increase in diabetes? It seems that the formation of oxidative stress is increased by hyperglycemia in diabetes and as a result, the antioxidant capacity changes play a significant role in increased apoptosis in testis of diabetic men [9].
Additionally, it has been reported that toll-like receptors (TLRs) and TRPV1 are the main receptors and canal, respectively, which participate in the induction of inflammation in the tissues which can lead to enhanced apoptosis [10,11]. Accordingly, it seems that the complications of diabetes in the testis can be associated with the expression of molecules. Therefore, a suitable therapeutic strategy can be considered for treatment of diabetes to reduce the risk of testis complication. Chemical or synthetic drugs have many side effects, so in many countries, traditional medicine, especially herbal medicines have been used to treat some of the complications of diabetes.
Zingiber Officinale Rosce is the scientific name of Ginger plant. Ginger is extensively used as a spice and food preservative in Southeast Asia, India and China. For many years, in countries like China and India ginger has been used to treat diseases such as nausea, stomach pain, gingivitis, tooth pain, digestive disorders, arthritis, respiratory disorders, atherosclerosis, migraine, depression, ulcers, high cholesterol, etc [12]. Thus, it seems that the herbal drug can be considered as an important candidate for treatment of diabetes to avoid testis complications. Accordingly, this study was aimed to evaluate the likely therapeutic effects of alcoholic extract of ginger on apoptosis of germ cells of in streptozotocin-induced diabetic rats as well as expression of apoptosis related molecules including Bax, Bcl-2, CyclinD1, TRPV1, TLR2 and TLR4.
Methods
Animals and experimental protocol
In this experimental study, male wistar rats at an average weight of 200-230 g were maintained in the animal house of the Faculty of Medicine, Rafsanjan University of Iran. All animals were maintained under controlled conditions of temperature (23 ± 2 °C) and light (12 h light/dark cycles). Animals received standard pellet food and water ad libitum. The experimental protocol was approved by the University Animal Care Committee. After weighing, rats were divided to five groups (n=12) as follows: non-treatment normal group (group 1), non- treatment diabetic group (sham group, group 2), ginger treatment diabetic group (group 3), insulin treatment diabetic group (group 4) and ginger-insulin treatment diabetic group (group 5). The first and second groups received saline only (equal volume). Diabetes was induced by injection of 60 mg/kg streptozotocin in the four remaining diabetic groups. After three days, blood was collected from the tip of the tail vein and Blood glucose levels were measured with a glucometer (Accucheck Glucose Meter, Roche, Switzerland). Animals with blood glucose levels of 220 mg/dl and above were taken into the study [13].
In addition, diabetic animals had symptoms such as polydipsia, polyuria, overeating. In addition to ginger, animals in the last group received insulin 4-6 units/kg each day. Ginger extract was injected (200 mg/kg every other day) in the two last groups. Ginger injections were done for 56 consecutive days. Twenty-four hours after the last injection, animals were anesthetized with ether and after dissecting the scrotum and other covers around the testis, both testis with tunica albuginea were removed. Testicles were used to evaluate the apoptosis by two different methods, Right testis for TUNEL method and left testis for real time-PCR.
Preparation of ginger extract
Ginger rhizome was prepared from Esfahan Plant Herbarium Company. Rhizome was powdered and equal volume of alcohol 50% was added to ginger powder. The mixture was kept at 70-80°C for 15 h in water bath. The suspension was centrifuged for 10 minutes at 2500 g. In order for all of the alcohol to evaporate, the supernatant was held in a water bath at 70-80°C. Finally, in order to obtain ginger stock solution, the appropriate amount of the originated extract was calculated and dissolved into the proportional volume of PBS. The stock solution was kept in a refrigerator until used [12].
In situ germ cell apoptosis detection
Right testicles were used to evaluate apoptosis by TUNEL method. The right removed testicles were put in bouin solution (as fixative) for 24 h. For deeper penetration of the fixative solution to the deep part of the testicles, each pole of testis was penetrated with a needle. To eliminate the yellow colour of picric acid from tissue, firstly, testicles were transferred to 70% ethanol for 2 days and alcohol was replaced 3 times each day. Secondly, tissues were transferred several times to a saturated solution of ethylic alcohol 70% and lithium carbonate. Thirdly, various stages of preparing tissue to sectioning such as dehydration, infiltration, embedding were done.
Finally, sections with a thickness of 7 μm were cut using a microtome. In situ cell death detection kit was purchased from Roche (Germany). Briefly, Germ cell apoptosis was examined as following: Seven micrometer thick sections were deparaffinized, rehydrated, washed and incubated with proteinase K. After adding H2O2 to quench peroxidase activity, again sections were incubated, firstly with the TUNEL reaction mixture [terminal deoxynucleotidyl transferase plus the nucleotide mixture in reaction buffer], and secondly with converter-AP [containing antifluorescein antibody (Fab fragment from sheep) conjugated with Alkaline Phosphatase (AP)], and thirdly with substrate solution [containing 7 mg diaminobenzidine (DAB) (Sigma) and 3 μl H2O2 in 10 ml of 50 mM Tris-HCl, pH 7.4]. Incubations were performed in moist chamber. Finally, samples were counterstained with Hematoxylin. Positive control sections were incubated with micrococcal nuclease or recombinant DNase1 to induce DNA strand breaks prior to labelling. Negative control sections were processed with label solution (without terminal transferase) [14,15].
Real time PCR
Left testicles were used to study apoptotic gene expression (Bax, Bcl-2, CyclinD1, TRPV1, TLR2 and TLR4) by real time PCR. The removed testicles were kept in sterile tubes containing ACSF at -80 until further processing. Then, testicles were homogenized in sterile condition and RNA was extracted to make cDNA. The β-actin gene was used as an internal control. The primers used have been shown in Table 1.
Total RNA was extracted from testis using Trizol Reagent (Invitrogen, Carlsbad, CA). The purity of extracted RNA was determined by electrophoresis on an ethidium bromide pre pretreated agarose gel along with measuring absorption by spectrophotometer and calculation of 260/280 ratio. The RNA was converted to cDNA using a cDNA synthesis kit (Bionner, Korea) with both oligo (dT) and random hexamer primers. The process of reverse transcription was performed by the following protocol: 70°C for 10 min (without reverse transcription enzyme), 20°C for 1 min (cooling step), addition of reverse transcription enzyme, 42°C for 60 min, and the protocol was completed by final step at 95°C for 10 min to terminate the activation of the reverse transcription enzyme.
Real-time PCR was performed using a SYBR green master mix (Bionner, Korea), combined with 200 mg of template cDNA with the appropriate primers (Table 1) in a Bio-Rad CFX96 system (Bio-Rad Company, USA) using the following program: 1 cycle of 95°C for 15 min, 40 cycles of 95°C for 30 s and 60°C for 30 s and finally, 72°C for 30 s. Primers were approved by Blast after design and synthesized by the Bionner Company (Korea).
| Bax (F) | GATGGCAACTTCAACTGGGG |
| Bax (R) | AGCCACCCTGGTCTTGGAT |
| Bcl-2 (F) | TGGCCTTCTTTGAGTTCGGT |
| Bcl-2 (R) | AGTTCCACAAAGGCATCCCAG |
| Cyclin D1 (F) | CAAGTGTGACCCGGACTGC |
| Cyclin D1 (R) | CACATCTCGCACGTCGGT |
| TRPV1 (F) | GACATGCCACCCAGCAGG |
| TRPV1 (R) | TCAATTCCCACACACCTCCC |
| TLR2 (F) | CTGATGGAGGTGGAGTTTGA |
| TLR (R) | TCCGTATTGTTACCGTTTCTA |
| TLR4 (F) | GAATTGTATCGCCTTCTTAG |
| TLR4 (R) | TGTGAGGTCGTTGAGGTTAG |
| Betta-Actin (F) | CTGTGCTGCTCACCGAGG |
| Betta-Actin (R) | CGGAGTCCATCACAATGCCT |
Table 1: The designed primers for the gene expression of Bax, Bcl-2, Cyclin D1, TRPV1, TLR2 and TLR4 in testis.
Real-time PCR was carried out in triplicate and the ß-actin was applied as a housekeeping gene for normalization of the amplified signals of the target genes. The sequences of the used primers are shown in Table 1. The quantity of cytokines mRNA in the testis was expressed as units relative to the amount of ß-actin mRNA. The dissociation stages, melting curves and quantitative analyses of the data were performed using CFX manager software version 1.1.308.111 (Bio-Rad, USA) [15-17].
Results
Our results show that the body weight decreased significantly in all diabetic groups at the end of study. In ginger insulin diabetic group, the weight of testis was the same as normal group, but in comparison to non-treatment diabetic group was significantly different (p ≤ 0.001) (Table 2).
| Group | Animal weight before starting the experiments | animal weight at the end of experiment | testis weight | testis weight/body weight ratio |
|---|---|---|---|---|
| 1 | 215.5 テつア 6.17 | 221.33 テつア 8.18 | 1.24 テつア 0.03 | 0.0056 テつア 0.00029 |
| 2 | 213.42 テつア 9.59 | 121.67 テつア 8.28 | 0.72 テつア 0.06 | 0.0061 テつア 0.00072 |
| 3 | 215.33 テつア 10.83 | 159.83 テつア 7.07*** | 0.77 テつア 0.09 | 0.0048 テつア 0.00067*** |
| 4 | 217.25 テつア 10.9 | 187.17 テつア 4.41*** | 0.80 テつア 0.09 | 0.0043 テつア 0.00050*** |
| 5 | 214.67 テつア 10.82 | 194.17 テつア 3.74*** | 1.18 テつア 0.05*** | 0.0062 テつア 0.00050 |
Table 2: Body and testis weights of animals in different studied groups.
Mean amount of calculated apoptosis index in the gingerinsulin treatment diabetic and non-treatment diabetic groups was 0.111 ± 0.008 and 0.180 ± 0.004 respectively, showing decreased apoptosis in the ginger-insulin treatment diabetic group in comparison with sham group after injections (p ≤ 0.05).
In this study, insulin is the mainstay of treatment, so if ginger is given alone it cannot be treated but if used with insulin it will be effective in reducing apoptosis and inflammation. Though it reduces apoptosis and inflammation but it is not significant. Apoptosis indices in non-treatment normal, ginger treatment and insulin treatment groups were 0.087 ± 0.003, 0.165 ± 0.007 and 0.0173 ± 0.002, respectively (Figures 1 and 2).
Figure 2: Effect of ginger on the apoptosis index in seminiferous tubules of rat testis. After treatment with saline, ginger, insulin and ginger-insulin together for 56 consecutive days, testes were sampled 24 h after the last treatment. As the figure illustrates, ginger plus insulin reduced the apoptosis index. Each bar represents the mean ± SE (n=12). *Significant differences between ginger-insulin treatment diabetic and non-treatment diabetic groups (P ≤ 0.05).
Our gene expression results showed that in the non-treatment diabetic group, the Bax gene was expressed less than other diabetic groups and there was a significant difference between mentioned group and ginger-insulin treatment diabetic group (p ≤ 0.01). As predicted, in the non-treatment normal group the expression of Bax gene (0.025 ± 0.010) was lower than other groups (Figure 3).
Figure 3: Effect of ginger on the expression of Bax gene in seminiferous tubules of diabetic rat testis. After treatment with saline, ginger, insulin and ginger-insulin together for 56 consecutive days, testes were sampled 24 h after the last treatment. Ginger plus insulin reduced the Bax gene expression. Each bar represents the mean ± SE (n=12). **Significant differences between ginger-insulin treatment diabetic and non-treatment diabetic groups (P ≤ 0.01).
The expression of Bcl2 mRNA was 1 ± 0.023 and 0.72 ± 0.032 for non-treatment diabetic and ginger-insulin treatment diabetic groups respectively, so the Bcl2 expression decreased significantly in ginger-insulin treatment diabetic group in comparison to non-treatment diabetic group (p ≤ 0.05) (Figure 4).
Figure 4: Effect of ginger on the expression of bcl2 gene in seminiferous tubules of diabetic rat testis. After treatment with saline, ginger, insulin and ginger-insulin together for 56 consecutive days, testes were sampled 24 h after the last treatment. Ginger plus insulin reduced the Bcl2 gene expression. Each bar represents the mean ± SE (n=12). *Significant differences between ginger-insulin treatment diabetic and non-treatment diabetic groups (P ≤ 0.05).
Unlike previous genes, the expression of CYC D1 gene increased significantly in ginger insulin treatment diabetic group in comparison to non-treatment diabetic group (p ≤ 0.05). The highest and lowest values obtained for this parameter were 0.76 ± 0.023 and 1.52 ± 0.042 respectively in non-treatment normal and ginger insulin treatment diabetic groups (Figure 5).
Figure 5: Effect of ginger on the expression of CYC D1 gene in seminiferous tubules of diabetic rat testis. After treatment with saline, ginger, insulin and ginger plus insulin together for 56 consecutive days, testes were sampled 24 h after the last treatment. Ginger plus insulin reduced the CYC D1 gene expression. Each bar represents the mean ± SE (n=12). *Significant differences between ginger-insulin treatment diabetic and non-treatment diabetic groups (P ≤ 0.05).
The results showed that mRNA levels of TRPV1 (P=0.026), TLR2 (P=0.026) and TLR4 (P=0.05) were significantly increased in the testis of the diabetic in comparison to normal healthy rats. The results also revealed that the expression of TRPV1 (P=0.006) and TLR2 (P=0.006) was significantly decreased in group 3 and 4 when compared with non-treated diabetic controls (Figures 6 and 7).
While only ginger in combination with insulin (group 5) can decrease mRNA levels of TLR4 (Figure 8).
Figure 8: Effect of ginger on the expression of TLR4 gene in seminiferous tubules of diabetic rat testis. The figure illustrates that mRNA levels of TLR4 were significantly increased in the diabetic rats and ginger, insulin and a combination of insulin-ginger reduced its expression. Each bar represents the mean ± SE (n=12).
As it is illustrated in the Figures 6-8, expression levels of TRPV1, TLR2 and TLR4 were not altered among other groups.
Discussion
Our results firstly, showed that the ginger extract could reduce, albeit at low level, apoptosis of sperm germ cells in diabetic groups, especially when ginger extract is used in combination with insulin. Secondly, increase of the CYCD1 gene expression in our results may represent a positive effect of extract on cell proliferation. According to the result of previous studies the ginger may be able to reduce germ cells apoptosis via decrease in blood sugar. The most important factor in herbal drugs in reduction of blood sugar is antioxidant compounds, but control of blood sugar alone is not able to prevent the diabetes complications [18]. With regard to the role of free radicals in diabetes, one of the areas of research in treatment of the disease is reduction of these radicals. In this context, the effect of antioxidants in preventing damages resulting from free radicals has been studies in diabetic diseases [3]. Since the oxidative stress results from an imbalance between the production of free radicals and reactive oxygen species, antioxidant defence mechanism must be able to reduce the harmful effects of these aggressive agents [15].
Therefore, an ideal drug for treatment of the diabetes must not only reduce the blood sugar level, but it should also have some antioxidant properties. To deal with free radicals and reactive oxygen species, chemical drugs which lower blood sugar should be able to control tissue damages in this disease, but they could also cause numerous side effects. Diabetes treatment without any side effects is still not completely possible and remains a challenge for the health system. Due to side effects of chemical synthetic drugs, researcher's attention has recently focused on the herbal drugs [19]. Diabetic diseases tend to use natural products, because the common hypoglycemic drugs have undesirable side effects. The potential role of medicinal plants as hypoglycemic agents has been seen in many botanical studies and the use of traditional medicinal plants has been reported in different cultures. In addition to being a good source of natural antioxidants, herbal medicines impose less financial pressure on the family economics of patients [20].
In Chinese and Indian ancient medicine, ginger is used to treat different diseases such as nausea, stomach and tooth pain, inflammation of gums, digestive and respiratory disorders, arthritis, atherosclerosis, migraine, depression, ulcer. Studies have shown that in addition to antioxidant and antiinflammatory properties, the ginger reduced the blood sugar with the antagonistic activity against serotonin receptors. It is known that treatment with antioxidants may improve glucose transport and its tolerance in patients with diabetes type 2 and animals with insulin resistance. Also, ginger with antioxidants including gingerols and shograns prevents the active metabolites of free radicals and eliminates these free radicals. Additionally, the results revealed that expression levels of TRPV1, TLR2 and TLR4 were significantly increased in group 2 (non-treated diabetic animals) in comparison to group 1 (healthy animals). Based on the fact that the molecules are potentially involved in the induction of inflammation, it appears that diabetes can enhance inflammation in the testis via several mechanisms including TLRs and also TRPV1 pathways. Interestingly, the results demonstrated that ginger and insulin were able to decrease expression levels of TRPV1 and TLR2. Based on the fact that TRPV1 is an important canal to induce inflammation and also TLR2 is a molecule with dual functions such as induction of inflammation, it may be concluded that ginger and insulin play critical roles in reduction of inflammation and can be used for inhibition of diabetes complication in the testis.
Additionally, due to the fact that, TLR4 uses both MYD88 and TRIF dependent pathways and based on the results which showed that only ginger in combination with insulin, but not in separate format, is able to decrease expression of TLR4, it appears that insulin and ginger are unable to decrease TLR4- related inflammation in separate administration. To the best of our knowledge, there are no related investigations regarding the roles of ginger and its combination with insulin on the expression of TLR2, but some investigations have evaluated the effects of ginger on the expression of TLR4 and TRPV1 in other animal models rather than diabetic animal model. For instance, a study revealed that ginger has an interaction with TRPV1 to decrease expression of IFN-γ, as inflammatory cytokines, by human T lymphocytes [21]. Another study reported that ginger can activate TRPV1 channels and consequently induce Ca² signals in the beta-cell which leads to sensitizing the beta-cells to glucose [22]. The effects of ginger on the expression and functions of TRPV1 have also been documented by several investigations [23,24]. Additionally, Ahn et al. showed that ginger inhibits homodimerization of TLR4 which suppress its functions [25]. Another study revealed that ginger suppresses expression of inflammatory genes in a NF-κB dependent manner [26]. NF-κB is a main transcription factor down-stream of TLR2 and 4 and participates in the expression of the TLRs [27-29].
Conclusion
Based on the results, it seems that ginger can be used as an important drug to decrease diabetes related inflammation in the testis.
Funding
This article has been supported by Rafsanjan University of Medical Sciences.
Acknowledgements
The current article is supported by a grant from Rafsanjan University of Medical Sciences.
References
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414-1431.
- Kim NN, Stankovic M, Cushman TT, Goldstein I, Munarriz R, Traish AM. Streptozotocin-induced diabetes in the rat is associated with changes in vaginal hemodynamics, morphology and biochemical markers. BMC Physiol. 2006;6:4.
- Mallick C, Mandal S, Barik B, Bhattacharya A, Ghosh D. Protection of testicular dysfunctions by MTEC, a formulated herbal drug, in streptozotocin induced diabetic rat. Biol Pharm Bull. 2007;30:84-90.
- Elyasi F, Kashi Z, Tasfieh B, Bahar A, Khademloo M. Sexual dysfunction in women with type 2 diabetes mellitus. Iran J Med Sci. 2015;40:206-213.
- Beatrice AM, Dutta D, Kumar M, Kumbenahalli Siddegowda S, Sinha A, Ray S, Chowdhury S. Testosterone levels and type 2 diabetes in men: current knowledge and clinical implications. Diabetes Metab Syndr Obes. 2014;7:481-486.
- Santos SA, Rinaldi JC, Martins AE, Camargo AC, Leonelli C, Delella FK, Felisbino SL, Justulin LA, Jr., Impact of gestational diabetes and lactational insulin replacement on structure and secretory function of offspring rat ventral prostate. Gen Comp Endocrinol. 2014;206:60-71.
- Giribabu N, Kumar KE, Rekha SS, Muniandy S, Salleh N. Chlorophytum borivilianum (Safed Musli) root extract prevents impairment in characteristics and elevation of oxidative stress in sperm of streptozotocin-induced adult male diabetic Wistar rats. BMC Complement Altern Med. 2014;14:291.
- Kilarkaje N, Al-Bader MM. Diabetes-induced oxidative dna damage alters p53-p21CIP1/Waf1 signaling in the rat testis. Reprod Sci. 2015;22:102-112.
- Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive
- function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008;4:46-54.
- Zare-Bidaki M, Tsukiyama-Kohara K, Arababadi MK. Toll-like receptor 4 and hepatitis B infection: molecular mechanisms and pathogenesis. Viral Immunol. 2014;27:321-326.
- Hakimizadeh E, Arababadi MK, Shamsizadeh A, Allahtavakoli M, Rezvani ME, Roohbakhsh A. Morphine Reduces Expression of TRPV1 Receptors in the Amygdala but not in the Hippocampus of Male Rats. Iran J Bas Med Sci. 2014;39:261-267.
- Ajith TA, Nivitha V, Usha S. Zingiber officinale Roscoe alone and in combination with alpha-tocopherol protect the kidney against cisplatin-induced acute renal failure. Food Chem Toxicol. 2007;45:921-927.
- Jafari Anarkooli I, Barzegar Ganji H, Pourheidar M. The Protective Effects of Insulin and Natural Honey against Hippocampal Cell Death in Streptozotocin-Induced Diabetic Rats. J Diabetes Res. 2014.
- Alavi SH, Taghavi MM, Moallem SA. Evaluation of effects of methamphetamine repeated dosing on proliferation and apoptosis of rat germ cells. Syst Biol Reprod Med. 2008;54:85-91.
- Li M, Liu Z, Zhuan L, Wang T, Guo S, Wang S, Liu J, Ye Z. Effects of apocynin on oxidative stress and expression of apoptosis-related genes in testes of diabetic rats. Mol Med Rep. 2013;7:47-52.
- Jiang X, Zhang C, Xin Y, Huang Z, Tan Y, Huang Y, Wang Y, Feng W, Li X, Li W, Qu Y, Cai L. Protective effect of FGF21 on type 1 diabetes-induced testicular apoptotic cell death probably via both mitochondrial- and endoplasmic reticulum stress-dependent pathways in the mouse model. Toxicol Lett. 2013;219:65-76.
- Jafarzadeh A, Azizi SV, Nemati M, Khoramdel-Azad H, Shamsizadeh A, Ayoobi F, Taghipour Z, Hassan ZM. Ginger Extract Reduces the Expression of IL-17 and IL-23 in the Sera and Central Nervous System of EAE Mice. Iran J Immunol. 2015;12:288-301.
- Amin A, Hamza AA, Kambal A, Daoud S. Herbal extracts counteract cisplatin-mediated cell death in rat testis. Asian J Androl. 2008;10:291-297.
- Khaki A, Khaki AA, Hajhosseini L, Golzar FS, Ainehchi N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr J Tradit Complement Altern Med. 2014;11:1-8.
- Shalaby MA, Hamowieh AR. Safety and efficacy of Zingiber officinale roots on fertility of male diabetic rats. Food Chem Toxicol. 2010;48:2920-2924.
- Schoenknecht C, Andersen G, Schmidts I, Schieberle P. Quantitation of Gingerols in Human Plasma by Newly Developed Stable Isotope Dilution Assays and Assessment of Their Immunomodulatory Potential. J Agricultural Food Chem. 2016;64:2269-2279.
- Rebellato P, Islam MS. [6]-shogaol induces Ca2+ signals by activating the TRPV1 channels in the rat insulinoma INS-1E cells. J Pancreas. 2014;15:33-37.
- Okumi H, Tashima K, Matsumoto K, Namiki T, Terasawa K, Horie S. Dietary agonists of TRPV1 inhibit gastric acid secretion in mice. Planta Med. 2012;78:1801-1806.
- Khan K, Singh A, Mittal M, Sharan K, Singh N, Dixit P, Sanyal S, Maurya R, Chattopadhyay N. [6]-Gingerol induces bone loss in ovary intact adult mice and augments osteoclast function via the transient receptor potential vanilloid 1 channel. Mol Nutr Food Res. 2012;56:1860-1873.
- Ahn SI, Lee JK, Youn HS. Inhibition of homodimerization of toll-like receptor 4 by 6-shogaol. Mol Cells. 2009;27:211-215.
- Lee HY, Park SH, Lee M, Kim HJ, Ryu SY, Kim ND, Hwang BY, Hong JT, Han SB, Kim Y. 1-Dehydro-[10]-gingerdione from ginger inhibits IKKbeta activity for NF-kappaB activation and suppresses NF-kappaB-regulated expression of inflammatory genes. Br J Pharmacol. 2012;167:128-140.
- Bagheri V, Askari A, Arababadi MK, Kennedy D. Can Toll-Like Receptor (TLR) 2 be considered as a new target for immunotherapy against hepatitis B infection? Hum Immun. 2014;75:549-554.
- Radman M, Golshiri A, Shamsizadeh A, Zainodini N, Bagheri V, Arababadi MK, Kennedy D. Toll-like receptor 4 plays significant roles during allergic rhinitis. Allergol Immunopathol (Madr). 2015;43:416-420.