- Biomedical Research (2016) Volume 27, Issue 2
The effect of adrenaline or noradrenaline with or without lidocaine on the contractile response of lipopolysaccharide-treated rat thoracic aortas.
| Shigeki Fujiwara1*, Akiko Noguchi2, Masanori Tsukamoto3, Shinichi Ito3, Uno Imaizumi1, Yoshinari Morimoto4, Kazu-ichi Yoshida1, Takeshi Yokoyama3 1Department of Anesthesiology, Graduate School of Dentistry, Kanagawa Dental University, Kanagawa, Japan 2Department of Anesthesiology and Critical Care Medicine, Saga University, Saga, Japan 3Department of Dental Anesthesiology, Faculty of Dental Science, Kyushu University, Fukuoka, Japan 4Department of Oral Science, Graduate school of Dentistry, Kanagawa Dental University, Kanagawa, Japan |
| Corresponding Author: Shigeki Fujiwara, Department of Anesthesiology Graduate School of Dentistry Kanagawa Dental University Japan |
| Accepted: January 30, 2016 |
Abstract
The analgesic effect of a local anesthetic is often insufficient in acutely inflamed tissues, due to the reduced pH and/or vasodilation. Thus, vasoconstrictors (adrenaline or noradrenaline) are often used to contain the anesthetic and prolong the analgesia, although there are minimal data regarding their effects in inflamed tissues. Therefore, we prepared a model of blood vessel inflammation (using lipopolysaccharide), and investigated the contractile effects of adrenaline or noradrenaline with and without lidocaine. Wistar rats’ thoracic aortas were cut into 3-mm-thick rings, which were stretched using a pair of hooks in an organ bath (Krebs-Henseleit solution, 37°C, pH=7.4). After lipopolysaccharide exposure (1 μg/mL), adrenaline or noradrenaline was applied in successive cumulative doses (10-9 to 10-5 M) with or without lidocaine (10-4 M), and the isometric vasocontractions were recorded. The lipopolysaccharide treatment attenuated the vasocontractions that were induced by adrenaline or noradrenaline with lidocaine in a time-dependent manner. Despite its vasodilation properties, lidocaine enhanced the contractile responses that were produced by low concentrations of adrenaline (10-8 to 10-7 M), which indicates that a sufficient analgesic effect depends on the concentration of lidocaine and adrenaline. Therefore, adrenaline should be used when injecting inflamed tissues with lidocaine.
Keywords |
||||||
| Lipopolysaccharide, Lidocaine, Vasoconstriction, Adrenaline, Noradrenaline | ||||||
Introduction |
||||||
| The administration of local anesthetics in acutely inflamed tissues often fails to achieve satisfactory analgesia, and several hypotheses have been proposed to explain this phenomenon, such as the effects of low pH or vasodilation in the inflamed area [1]. In this context, vasodilation may increase the local blood flow, resulting in the rapid removal of the local anesthetic from the injection site. Although vasoconstrictors are typically used with local anesthetics to prolong the duration of anesthesia, their effects (when combined with local anesthetics) on the dilated vessels in inflamed tissues are not well understood. Therefore, to investigate the effects of vasoconstrictors and a local anesthetic on inflamed vessels, we measured the contractile responses of inflamed rat thoracic aortas to Adrenaline (AD) or Noradrenaline (NA) that were administered with or without lidocaine. | ||||||
Materials and Methods |
||||||
Animals |
||||||
| Sixteen male Wistar rats (6-8 weeks old) were used in this study. The protocol for this study was reviewed and approved by the institutional animal care committee of Asahi University (Gifu, Japan). | ||||||
Functional experiments |
||||||
| The rats were anesthetized via inhalation of sevoflurane and killed via decapitation. The thoracic aortas were then isolated for the functional experiments, and 3-mm rings were carefully prepared from the thoracic aortas under a dissecting microscope, as previously described [2,3]. | ||||||
| The thoracic aorta rings were placed in Krebs-Henseleit solution (KRB; pH 7.4) that contained 118 mM NaCl, 4.7 mM KCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 1.2 mM MgSO4, 2.5 mM CaCl2, and 11.5 mM glucose. Each ring was carefully suspended in an organ chamber containing 5 mL of KRB that was bubbled with 95% O2/5% CO2 at 37°C, and the changes in tension were recorded isometrically. A resting tension of 1.0 g was applied, and the rings were allowed to equilibrate for 60 min, with readjustment of the passive tension when needed. | ||||||
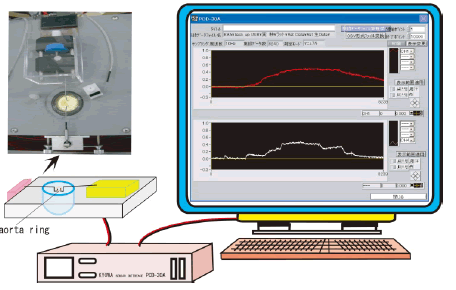
| During this period, the rings were washed every 20 min with fresh bubbled and warmed KRB. The changes in isometric vasocontractions were recorded continuously using an amplifier system and the PCD-30A computer system (KYOWA, Japan) (Figure 1). Vasoconstrictions were quantified as the contractile force (mg) divided by the wet tissue weight (mg). The methods for these experiments were developed based on the reported methods of Yoshida et al. and Kobayashi et al. [4,5]. | ||||||
Treatment with lipopolysaccharide |
||||||
| The rings were incubated with KRB that contained Lipopolysaccharide (LPS) (1 μg/mL) at 37°C for 6 h. Under these conditions, AD and NA were applied in a cumulative manner (10-9 M to 10-5 M) with or without lidocaine (10-4 M). | ||||||
Chemicals |
||||||
| The AD, NA, and lidocaine were purchased from Sigma (St. Louis, USA), and the LPS was purchased from Invitrogen (California, USA). All other reagents were of analytical grade. | ||||||
Statistical analysis |
||||||
| All data were expressed as mean ± standard error. The Mann- Whitney U test was used to compare two groups, and the Steel- Dwass test was used to compare several different groups. Differences with a P-value of <0.05 were considered statistically significant. | ||||||
| All analyses were performed using SPSS software (version 16.0; SPSS Inc., Chicago, IL) and Ekuseru-Toukei 2006 software (Social Survey Research Information Co. Ltd., Japan). | ||||||
Results |
||||||
Contractile responses of non-LPS-treated aortas to AD and NA in the absence of lidocaine |
||||||
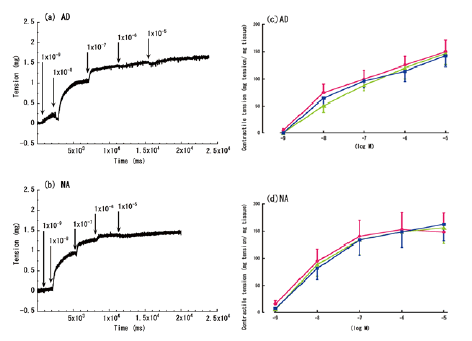
| The aorta rings’ contractile responses to varying concentrations of AD or NA, without lidocaine and LPS treatment, are shown in Figure 2a and 2b. Gradual increases in the AD and NA concentrations produced gradual responses in the isolated rat aortas, with the greatest response observed at concentrations of 10-5 M. Both AD and NA evoked concentration-dependent contractile responses, which stabilized after 6 h (Figure 2c and 2d). | ||||||
Contractile responses of non-LPS-treated aortas to AD and NA in the presence of lidocaine |
||||||
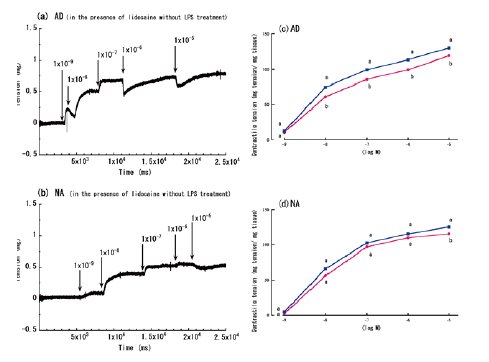
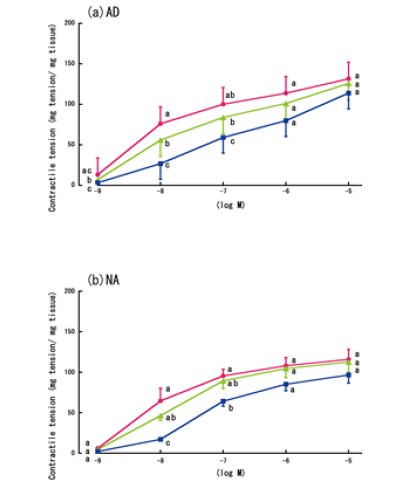
| Figure 3a and 3b shows the aorta rings’ contractile responses to varying concentrations of AD or NA, in the presence of lidocaine and without LPS treatment. Similar to the experiment without lidocaine, the aorta contractile responses in these conditions were the greatest at concentrations of 10-5 M. The addition of lidocaine (10-4 M) created a small, but significant, enhancement in the contractions that were induced by most concentrations of AD (10-8-10-5 M) (Figure 3c), although lidocaine only significantly enhanced the contractions that were induced by the highest concentration of NA (10-5 M) (Figure 3d). | ||||||
Contractile responses of LPS-treated aortas to AD and NA in the absence of lidocaine |
||||||
| Figure 4 shows the LPS-treated aorta rings’ time-dependent contractile responses to AD or NA. Treatment with LPS significantly diminished the contractile responses to AD or NA at concentrations of 10-8 M. However, the differences in the responses were not significant at the highest concentration of AD or NA (10-5 M) (Figure 4a and 4b). | ||||||
Contractile responses of LPS-treated aortas to AD and NA in the presence of lidocaine |
||||||
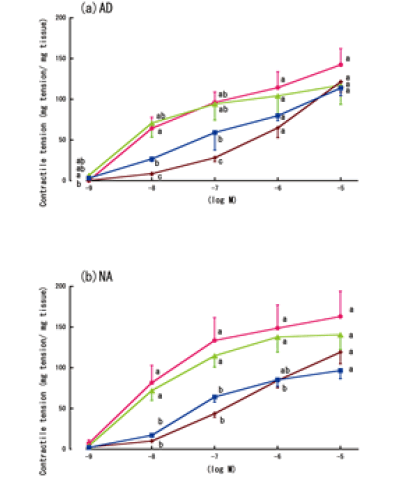
| Figure 5 shows the LPS-treated aorta rings’ time-dependent contractile responses to AD or NA. Only AD treatment at a concentration of 10-8 M resulted in significant differences in the responses over time. However, no significant differences in the responses over time were observed at AD or NA concentrations of 10-6-10-5 M (Figure 5a and 5b). | ||||||
Contractile responses of aortas to AD and NA according to LPS and lidocaine treatment |
||||||
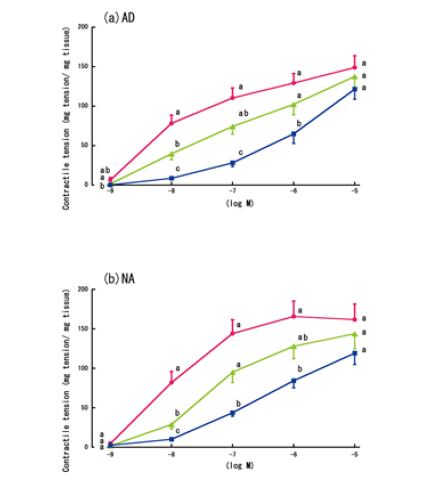
| The contractile responses to AD or NA, with or without the 6-h LPS treatment, and with or without lidocaine, are shown in Figure 6. Although lidocaine reduced the non-LPS-treated aorta rings’ contractile responses to high concentrations of AD (10-5 M), this reduction was not statistically significant. In contrast, lidocaine partially increased the contractile responses of the LPS-treated aorta rings to low concentrations of AD (10-8 M to 10-7 M) (Figure 6a). However, lidocaine did not significantly affect the LPS-treated aorta rings’ contractile responses to NA (Figure 6b). | ||||||
Discussion |
||||||
| In this study, we investigated whether AD or NA would optimize the effect of a local anesthetic that was injected into inflamed tissue. Using a model of LPS-induced aortic inflammation, we evaluated the alteration of the contractile responses to AD or NA in the presence or absence of lidocaine. Our results indicate that, although the responses to both vasoconstrictors decreased after LPS treatment, the contractility that was evoked by AD was partially maintained in the presence of lidocaine. Interestingly, LPS treatment is thought to reduce vessels’ contractile responses through the increased expression of inducible Nitrous Oxide Synthase (iNOS) in the vascular smooth muscle, or through the massive production of Nitrous Oxide (NO) in the vascular endothelium [6-8]. If this hypothesis is correct, our data indicate that iNOS expression reaches a maximum within 6 h after the addition of LPS (Figure 4 and Figure 5). Therefore, AD may help to modulate the LPS-induced effects on iNOS/NO in inflamed aortas, and thereby preserve the contractile response in these tissues. | ||||||
| Although the aortas’ vasoconstrictive response to low concentrations of AD was decreased after LPS treatment, the response was partially preserved by the addition of lidocaine. Lidocaine is known to constrict vessels at low concentrations, and to relax vessels at high concentrations [9-11]. Although lidocaine at a concentration of 10-4 M had a minor effect on the observed contractile response to AD, the vasoconstrictive action of lidocaine itself may have been involved in this finding. Furthermore, in the LPS-treated aortas, lidocaine partially increased their contractile responses to a low concentration of AD (10-8 M), which is equivalent to the concentration for general clinical use (approximately 1:80,000-200,000 for 1-2% lidocaine with AD; Figure 3c and Figure 6a). Therefore, in patients who are receiving the standard concentration of AD, the use of lidocaine may help to preserve the analgesic effect in inflamed tissues. | ||||||
| This study includes several limitations that warrant consideration. First, our findings are limited by the use of rat thoracic aortas rings, which were not under the control of the autonomic nervous system. However, it is thought that the peripheral vessels (arterial/venous) are sufficiently contracted when using lidocaine with 1:80,000-200,000 AD that is injected into rat and human local tissue [12,13]. Second, we used lidocaine at a concentration of 10-4 M, which is approximately equivalent to 0.01% lidocaine, as it was the maximum concentration for which the contractile response of the rat aortas did not disappear (data not shown). Third, the pH within inflamed tissues is known to be <7.4, while our results were obtained at a pH of 7.4. Fourth, we did not observe any clear suppression of the vascular contractile response to NA when the aortas were treated using lidocaine, although a previous report has indicated that lidocaine suppresses the vasoconstrictive activity of NA [14]. In this study, our data indicate that lidocaine significantly suppresses the vascular contractile response to NA, except for at a high concentration (10-5 M) (Figure 3d), although lidocaine did not clearly affect the LPS-treated vessels’ contractile responses to NA (Figure 6b). Fifth, we tested the endothelium-dependent vasorelaxation in vessels that were preconstricted using phenylephrine based on the response to the muscarinic receptor agonist (acetylcholine) [15,16]. Thus, we did not measure NO concentrations in the present study, and this parameter should be evaluated in future studies. | ||||||
| In the present study, we hypothesized that the local tissues received blood from the peripheral artery. Furthermore, we hypothesized that the anesthesia effect of lidocaine would be affected by the constriction and relaxation of the peripheral arterial. In this context, it would be valuable to examine this mechanism for the arteries around the oral cavity, although those specific arteries have not been evaluated in detailed studies. Therefore, we used the rat aorta in the present study, as it has been used in numerous other model-based studies, and its properties are clearly understood. Thus, future studies are needed to evaluate our findings in the clinical setting (e.g., for the inferior alveolar artery and lingual artery). | ||||||
Conclusion |
||||||
| We developed a model of LPS-induced vessel inflammation to test the effects of AD or NA, which were administered with or without lidocaine. Our results indicate that the LPS-treated vessels’ contractile responses to low concentrations of AD were partially maintained in the presence of lidocaine. Therefore, we conclude that AD is the optimal vasoconstrictor to prolong the lidocaine-induced analgesic effect in inflamed tissues. | ||||||
Acknowledgement |
||||||
| A part of this study was presented at Euroanaesthesia 2013 (Barcelona, Spain, June 2013). We thank Shuhei Kurashige and Ko Takakura for their technical assistance, and Editage for editing the manuscript. Asahi University provided financial support. | ||||||
Figures at a glance |
||||||
|
||||||
|
||||||
References |
||||||
|