Research Article - Biomedical Research (2017) Volume 28, Issue 14
The difference of organism redox state in AIDS combined with tuberculosis and tuberculosis only patients
Qingxi Wang* and Zemin Wang
Department of Clinical Laboratory, Ji'nan Central Hospital, PR China
- *Corresponding Author:
- Changmei Wang
Department of Clinical Laboratory
Ji'nan Central Hospital, PR China
Accepted on June 6, 2017
Abstract
Background: To compare the differences of organism redox state in AIDS patients complicated with tuberculosis and tuberculosis patients.
Methods: Subjects were divided into two groups, AIDS and TB group (n=40) and TB group (n=40) with 30 healthy subjects as the control group. Based on the measurement of reduced Glutathione (GSH) and oxidized glutathione (GSSG) as well as NADPH and NADP, the ratios of GSH/GSSG and NADPH/ NADP were used to describe the redox state of patients and compare the organism redox state in two groups.
Results: The levels of GSH, GSSG, GSH/GSSG, NADPH, NADP+, NADPH/NADP+ in AIDS and TB group, TB group and control group were different (F=115.0, P<0.01, F=3944.0, P<0.01, F=1957.0, P<0.01, F=318.0, P<0.01, F=318.0, P<0.01, F=12.0, P<0.01, F=104.0, P<0.01).
Conclusion: There is a difference in the redox state of AIDS patients complicated with tuberculosis and tuberculosis patients.
Keywords
AIDS, Tuberculosis, AIDS combined with tuberculosis, Organism redox state.
Introduction
Tuberculosis is still an infectious disease threatening the health of the people. In 2015, 10.4 million new tuberculosis patients were reported, of which 1.2 million were tuberculosis patients with HIV infection, accounting for 11% of all TB patients [1]. Tuberculosis was the most common opportunistic infections in AIDS patients with the mortality rate of 30% [2]. Therefore, the new approach to the treatment of AIDS combined with tuberculosis has become a new hot spot in clinical research. Studies have shown the presence of oxidative stress in patients with tuberculosis [3]. Mycobacterium tuberculosis (Mtb) suffered from exogenous oxidative stress [4]. In the course of infection, mycobacteria were affected by lots of redox stresses, such as Reactive Oxygen Species (ROS), Reactive Nitrogen Species (RNS) and hypoxia [5]. Toxicological effect induced by ROS was further compounded in Mtb result from the absence of important DNA repair pathways [6,7]. In addition, ROS and RNS produced by host was the key to control Mtb [8,9]. Moreover, our preliminary study also showed that the redox state of AIDS patients was biased toward the oxidation direction. Some researchers suggested that oxidative stressinduced free radicals could lead to cellular damage in HIV [10,11]. And Pocernich et al. [12] showed that AIDS dementia was related to increased oxidative stress. Furthermore, TB is the main infectious cause of death in HIV co-infected patients [13]. However, there were little relevant reports on the difference between the redox state of AIDS patients with tuberculosis and tuberculosis patients. In this study, 40 cases of AIDS patient with tuberculosis and 40 cases of tuberculosis patients were tested on redox state to compare the difference.
Materials and Methods
Instruments
Refrigerated centrifuge (20PR-52D, Japan HI-TACHI); ultralow temperature storage box (MDF-382E, Japan Sanyo); centrifuge (TDL-5-A, Shanghai Anting); high-speed refrigerated centrifuge (1-15K, United States Sigma).
Reagents
Reduced glutathione (GSH) and oxidized glutathione (GSSG) kit (Wuhan Biofavor, product code S0053); Nicotinamide adenine dinucleotide phosphate (NADP+) and NADPH kit (Wuhan Biofavor). In accordance with the requirements of the kit instructions, related reagents were prepared.
Object and grouping
The subjects of AIDS and TB group were selected from patients treated in Beijing Medical Center from June 2010 to October 2016 (A total of 40 patients, aged from 18 to 59 y old, participated in the group, which had 30 males and 10 females with the average age of 42.5 y old). AIDS diagnostic criteria referred to Guidelines for diagnosis and treatment of HIV/ AIDS in China [14]. Anti-HIV antibody test was conducted by Wuhan City Center for Disease Control and Prevention. Tuberculosis diagnosis referred to tuberculosis-diagnosis, management, prevention, and control guidance [15]. The subjects of TB group (TB group were selected from patients treated in Beijing Medical Center from June 2010 to October 2016 (Methods 40 subjects, 30 males and 10 females, aged from 18 to 60 y old with the average age of 43.4, were included in the this group). Thirty healthy subjects were selected as the control group.
Research methods
The fasting venous blood samples (3 ml) were collected and stored in a cryopreservation chamber after low-temperature centrifugal in heparin anticoagulant tube at -70°C for further NADPH/NADP+ and GSH/GSSG measurement. All samples were re-dissolved at room temperature before the determination. According to the requirements of kit, GSH and GSSG levels were measured by colorimetry. NADPH and NADP+ were measured by enzyme-linked immunosorbent assay.
Statistical processing
SPSS 11.5 statistical analysis software package was used for statistical analysis of data, and measurement data was recorded as x̄ ± s. T test was used to compare between groups. A statistical significance was defined when p<0.05.
Results
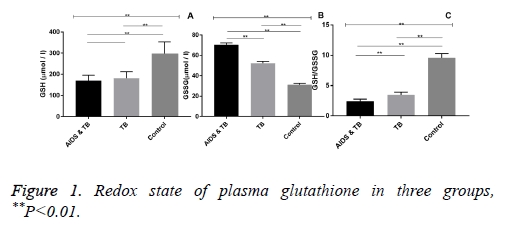
Redox state of plasma glutathione
The levels of GSH in AIDS and TB group, TB group and Control group were different (F=115.0, P<0.01). Compared with control group, the GSH levels of AIDS and TB group and TB group were significantly lower (P<0.01). The levels of GSSG in AIDS & TB group, TB group and control group were different (F=3944.0, P<0.01). Compared with control group, the GSSG levels in AIDS and TB group and TB group were significantly lower (P<0.01). Compared with TB group, the GSSG level of AIDS and TB group was significantly increased (P<0.01). The GSH/GSSG values of AIDS and TB group, TB group and Control group were different (F=1957.0, P<0.01). Compared with control group, the GSH/GSSG values of AIDS and TB group and TB group were significantly decreased (P<0.01). Compared with TB group, the GSH/GSSG value of AIDS and TB group was significantly decreased (P<0.01, Figure 1).
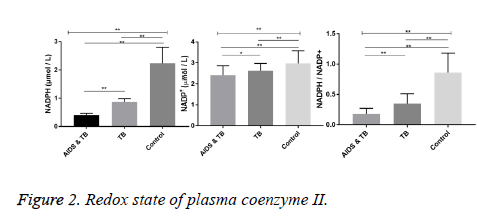
Redox state of plasma coenzyme II
The levels of NADPH in AIDS and TB group, TB group and Control group were different (F=318.0, P<0.01). Compared with the control group, the levels of NADPH in AIDS and TB group and TB group were significantly decreased (P<0.01). The levels of NADP+ in AIDS and TB group, TB group and control group were different (F=12.0, P<0.01). Compared with the control group, the levels of NADP+ in AIDS and TB group and TB group were significantly decreased (P<0.01). Compared with TB group, the level of NADP+ in AIDS and TB group was significantly decreased (P<0.01). The NADPH/ NADP+ ratio of AIDS and TB group, TB group and control group was different (F=104.0, P<0.01). Compared with the control group, the ratios of NADPH/NADP+ in AIDS and TB group and TB group were significantly decreased (P<0.01). Compared with TB group, the ratio of NADPH/NADP+ in AIDS and TB group was significantly decreased (P<0.01, Figure 2).
Discussion
HIV infection is the most important risk factor for tuberculosis. Tuberculosis is the most common opportunistic infection of HIV/AIDS, which can lead to a significant increase in the probability and mortality of other opportunistic infections in AIDS patients. Studies have found that the redox states of HIV/AIDS patients were biased toward the oxidation direction [16,17], and its degree of bias was positively correlated with disease progression. GSH is the main defensive mechanisms against toxic agents and oxidant-mediated injury [18,19]. And the GSH/GSSG ratio is a useful measure of cellular redox status [20]. In addition, NADPH/NADP+ oxidation and reduction played an important role in maintaining GSH/GSSG equilibrium [21]. With Intracellular GSH depletion, the incidence of tuberculosis in AIDS patients was much higher than healthy individuals. The study showed that the GSH levels of peripheral monocytes and erythrocytes in tuberculosis patients were significantly decreased [22]. Therefore, no matter tuberculosis or AIDS patients, the occurrence and development of disease were related to the body redox situation. This study showed that, based on GSH or NAD-PH concentration, redox state were biased toward the oxidation direction. And patients with AIDS combined tuberculosis showed more obvious bias than tuberculosis patients, resulting from overlay effect of AIDS and tuberculosis. The change of redox state was one of the important mechanisms of AIDS and had a certain relevance to AIDS development. This study provided a basis for the treatment of AIDS patients with tuberculosis from the perspective of changing the body's redox state.
References
- World Health Organization (WHO). Global tuberculosis report 2014.
- Walt MVD, Lancaster J, Shean K. Tuberculosis case fatality and other causes of death among multidrug-resistant tuberculosis patients in a high HIV prevalence setting, 2000-2008, South Africa. Plos One 2016; 11: 0144249.
- Wagh V, Rajopadhye S, Mukherjee S, Urhekar A, Modi D. Assessment of oxidative stress in serum of pulmonary tuberculosis patients. Int J Res Med Sci 2016; 4: 3328-3332.
- Kumar A, Farhana A, Guidry L, Saini V, Hondalus M. Redox homeostasis in mycobacteria: the key to tuberculosis control? Expert Rev Mol Med 2011; 13: 39.
- Bhat SA, Singh N, Trivedi A, Kansal P, Gupta P. The mechanism of redox sensing in Mycobacterium tuberculosis. Free Radic Biol Med 2012; 53: 1625-1641.
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998; 393: 537-544.
- Kurthkoti K, Varshney U. Distinct mechanisms of DNA repair in mycobacteria and their implications in attenuation of the pathogen growth. Mech Ageing Dev 2012; 133: 138-146.
- Ehrt S, Schnappinger D. Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell Microbiol 2009; 11: 1170-1178.
- Cooper AM, Segal BH, Frank AA, Holland SM, Orme IM. Transient loss of resistance to pulmonary tuberculosis in p47 (phox-/-) mice. Infect Immun 2000; 68: 1231-1234.
- Turchan J, Pocernich CB, Gairola C, Chauhan A, Schifitto G. Oxidative stress in HIV demented patients and protection ex vivo with novel antioxidants. Neurology 2003; 60: 307-314.
- Aukrust P, Muller F, Svardal AM. Disturbed glutathione metabolism and decreased antioxidant levels in human immunodeficiency virus-infected patients during highly active antiretroviral therapy-potential immunomodulatory effects of antioxidants. J Infect Dis 2003; 188: 232.
- Pocernich CB, Sultana R, Mohmmad-Abdul H. HIV-dementia, Tat-induced oxidative stress, and antioxidant therapeutic considerations. Brain Res Rev 2005; 50: 14.
- Hesseling AC, Rabie H. Tuberculosis and HIV remain major causes of death in African children. J Int Tubercul Lung Dis 2016; 20: 996.
- Chinese Medical Association; Chinese Center for Disease Control and Prevention. Guidelines for diagnosis and treatment of HIV/AIDS in China, 2005. Chin Med J (Engl) 2006; 119: 1589-1608.
- Hoppe LE, Kettle R, Eisenhut M, Abubakar I. Tuberculosis Diagnosis, Management, Prevention, and Control: Summary of Updated Nice Guidance. BMJ 2016; 352: 6747.
- Tasca KI, Caleffi JT, Correa CR, Gatto M, Tavares FC. Antiretroviral therapy initiation alters the redox system of asymptomatic HIV-infected individuals: A Longitudinal Study. Oxid Med Cell Longev 2017; 2017: 1-10.
- Ivanov AV, Valuevelliston VT, Ivanova ON, Kochetkov SN, Starodubova ES, Bartosch B, Isaguliants MG. Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev 2016; 2016: 8910396.
- Catarzi S, Favilli F, Romagnoli C, Marcucci T, Picariello L. Oxidative state and IL-6 production in intestinal myofibroblasts of Crohns disease patients. Inflamm Bowel Dis 2011; 17: 1674-1684.
- Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal 2005; 7: 42-59.
- Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol 2002; 348: 93-112.
- Yap LP, Garcia JV, Han D, Cadenas E. The energy-redox axis in aging and age-related neurodegeneration. Adv Drug Deliv Rev 2009; 61: 1283-1298.
- Mohammadien H, Mohamad A, Abdel-Aziz A. Immunophenotypic characterisation of peripheral blood mononuclear cells in patients with Mdr pulmonary Tb. Chest 2016; 150: 212.