- Biomedical Research (2012) Volume 23, Issue 4
The body wall crude extract of Stichopus variegatus promotes repair of acute contused spinal cord injury in rats by improving motor function and reduces intramedullary hemorrhage
Azim Patar1, Hasnan Jaafar2, Syed Mohsin Syed Sahil Jamalullail3, Jafri Malin Abdullah1*1Department of Neurosciences, Universiti Sains Malaysia,16150 Kota Bharu, Kelantan, Malaysia
2Department of Pathology, Universiti Sains Malaysia,16150 Kota Bharu, Kelantan, Malaysia
3Biomedical & Health Research Platform, Universiti Sains Malaysia,16150 Kota Bharu, Kelantan, Malaysia
- *Corresponding Author:
- Jafri Malin Abdullah
Department of Neurosciences
School of Medical Sciences
Universiti Sains Malaysia
6150 Kota Bharu Kelantan
Malayasia
Accepted date: May 25 2012
Abstract
We investigated whether Malaysian Sea cucumber, Stichopus variegatus (SV) extracts promoted repair in acute contused spinal cord injury in a rat model. Adults female Sprague Dawley rats weighted at 200-250g were subjected into seven groups. Rats had 10g rod dropped from heights of 25mm onto exposed cord at T9/T10 using NYU impactor device. Locomotor recovery was measured using Basso Beatie Breshnan (BBB) Locomoting scale after two weeks of intrathecal intervention with different doses of SV crude extracts, methylprednisolone and control groups. The methylprednisolone and 10 μg/kg SV treated groups showed a significance difference of BBB scores compared to control group indicating the improvement of motor functions after 14 days of injury(p<0.05). The intramedullary hemorrhage was found affected gray matter than white matter after contusion injury(p<0.01). However, methylprednisolone and 10 μg/kg SV extracts reduces intramedullary hemorrage compare to control group (p<0.05). Correlation coefficient analysis demonstrated a significance negative correlation between BBB scores and gray matter hemorrhage(r2= -0.99) and white matter hemorrhage (r2= -0.93). These findings lead to a conclusion that severity of hemorrhage was associated with behavior deficits. We also found Methylprednisolone and 10 μg/kg SV extracts spared the white matter (74.2% and 67.7% respectively) after 14 days of injury compared to control group (33%). In this present study, the white matter sparing was directly proportional to behavior deficits (r2= 0.91).Therefore, 10 μg/kg of SV extracts in this study strengthens the hypothesis the S. variegatus body wall extract is showed the capacity of promoting repair by improving motor functions and reduces intramedullary hemorrhage of acute contused spinal cord injury in rats.
Keywords
Stichopus variegatus, rat contusion spinal cord injury, white matter sparing, intramedullary hemorrhage, intrathecal
Introduction
Historically and continually eversince the time immemorial, natural products have been extensively utilized by the locals as home-based remedies to treat a wide range of ailments.Sea cucumbers from Malaysian Perhentian Islands were well known as potential for commercial value as well as edible food source and posses medicinal value to the locals [1]. The species of study, Stichopus variegatus (also known as Stichopus hermanii) not only serves as food source but it offers a variety of remedies which have been proven in an animal model system [2]. Apart from that, S. variegatus water extract (SVWE) has been reported to be used for rheumatoid arthritis [3], abdominal pain, liver damage [4] as well as heart ailments [5]. Numerous experiments that has revealed various in vivo effects such as antioxidant experiment [6], anti-angiogenosis [7], anti-microbacterial effect [1], potential tissue repairs[8], antithrombotic effects[5,9] and its proliferation effect on neurosphere [10]. However, to the best of our knowledge, study aimed to elucidate the reputed efficacy of this species particulary on behaviour of spinal contusive rat, hemorrhage and myelin spared in acute experimental settings is remains unexplored.There is no scientific report on the effects of SVWE on experimental acute spinal cord injury in rat models.
Acute spinal cord injury undergoes sequel pathologic changes including hemorrhagic, edema, axonal and neuronal necrosis as well as demyelination followed by cyst formation and infarction of spinal tissues [11]. Petechial hemorrhages occurred in the gray matter and edema of the white matter after 15 minutes of acute injury. The hemorrhages were increased after 2 hours, 4 hours, 6 days and worsen with time leading to necrosis [12]. It progressed into different area that correspond to the epidural, subdural, subarachnoid and intramedular spaces.
The amount of spared spinal cord tissues , particularly white matter in animal studies has been shown to have correlation with behavioural recovery after spinal cord injury [12]. Young and co-workers (1993) have demonstrated the recovery of motor function to a normal functional level after spinal cord injury in rats is small increment as 4-6% of the cortical motor neuron regains physiologic connection through the injured spinal cord at the caudal level [13]. Basso et al. (1996) have also shown that the sparing tissues (5%) of the fibers at lesion centre are sufficient to help in production of basic locomotion observed following spinal cord contusion injury in rats [14,15].
The present study was undertaken to investigate the effect of intrathecal SV extracts treatments on locomotor recovery, intramedullary hemorrhage and white matter sparing on contused rat spinal cord injury.
Materials and Methods
The methodology that involved in investigating the effect S. variegatus(SV) crude extracts on an acute spinal cord injury rats can be summarized into six (6) sections: SV water extraction, contusion animal model, intrathecal infusion of the SV crude extracts, post-operative care, behavioral tests and histology analysis.
SV Water Extraction
The extraction of the raw powder was detailed explained on our previous report [8].Briefly, a 20 g of homogenized tissue was mixed with 100ml of distilled water. The mixture was shaken at 80 strokes / min at room temperature for 3 hours. All the supernatants were combined in the round bottom flask for freeze dried process.The powdery-like extracts were subjected to sterilization using high electron beam rays according to [16]. The sterilization was performed using Linear Accelerator (Siemens Primus, Germany) and subjected to gamma rays at 50 Gray (Gy) for 10min time exposure with in a 5 cm depth in distilled water as adapted from Furuta et al., (2002).
Animals
A total of seventy of rats (n=70) were used in this study. Adult female Sprague Dawley rats weighted at 200-250g were subjected into seven groups respectively. The animals were purchased from the Animal Research And Service Centre(ARAS), Universiti Sains Malaysia. All the rats were subjected to surgical procedures according to the approval of Universiti Sains Malaysia Animal Ethics Committee with Animal Ethics reference number: USM/Animal Ethics Approval/2008/(35)(113). The animals were housed singly in Polypropylene cage (425 x 266 x 185 mm) (Techniplast, Italy) with wood shavings (Whitten Molen, Netherlands), the lighting was controlled on 12 hour light and 12 hour dark cycle. The animals had access to food and water ad libitium.
Thoracic 9 (T9) & Thoracic 10 (T10) laminectomy
The rats were anaesthesized with Ketamine (80mg/kg) (Troy Laboratories Pty Limited, Australia) and Xylazine (10mg/kg) (Troy Laboratories Pty Limited, Australia). The skin was shaved at the dorsal midline area and scrubbed with Povidone Iodine, 70% Etanol and Povidone Iodine (three times each). An ophthalmic lubricant (Ophtrex Ltd, England) was applied to both eyes of animal to avoid dehydrated during surgery. A skin incision was made along the midline of the dorsal under sterile aseptic techniques. The laminae and transverse process of T9 and T11 were exposed by gentle blunt dissection of paravertebral muscles.A T5 vertebrae was identify via locating the azygous vein which was the prominent vessel within multilocular adipose tissue, interscapular hibernating gland [17]. This anatomical landmark is found after caudal extremity of the interscapular hibernating gland was lifted underlying muscles. The back edge of scalpel blade was used to determine the T5 process which located under the vessels. Later, the process was continued counted in a caudal direction to T8, T9, T10 and T11.
Contusion Injury
Contusion injury was induced on spinal cord of the rats using New York University (NYU) Impactor device (WM Keck Center for Collaborative Neuroscience, USA). The contusion was performed upon completion of laminectomy. In this study, the rod was dropped from heights of 25mm for moderate contusion injury [15,18,19].The procedures were followed according to [20,21]. Briefly, the animal was positioned on the platform under the vertebral clamps. The rostal clamp was allowed to clamp T8 spinous process and the caudal clamped over T11 spinous process. The ground wire was clamped to adjacent tissue to complete the NYU system circuits.
Intrathecal infusion of SV crude extracts
After the contusion injury, the intrathecal surgery was performed; the partial laminectomy was done at the T12 and T13 vertebral junction [22,23]. The paraspinous muscles attached to T13 were removed and a small incision was made in the lateral dura. The 1.5 cm length of PE60 tubing of iPRECIO™ (PrimeTech Corporation, Japan) was inserted slowly under the T13 up to T10. The cerebrospinal fluid from the laminectomy space was removed using patties. The muscles were sutures over laminectomy site in layers using (Cat Gut® 6/0, England). The iPRECIO™ pumps (PrimeTech Corporation, Japan) was secured by suturing to superficial muscles using (Cat Gut® 6/0, England). The usage of iPRECIO™ pumps followed the manufacturers instruction for filling and priming [24].The skin incision was closed with absorbable suture, Dafilon® 6/0 (B|Braun, Germany). The animals were given 10 cc of 0.9% sterile saline subcutaneously and 0.1 cc of Gentocin (Vedco Inc.,USA) was given intramuscularly. All the surgical procedures were performed under microsurgical microscope (Zeiss, Germany).
Post Operative Care
The post operative care was slightly followed [22,23,25,26]. Briefly, during less than 24 hours immediate recovery care, the animal was injected subcutaneously with 5cc 0.9% saline (B|Braun, Germany). Then, the animal was placed in heating pad inside the clean cage and was wrapped with the towel. The heating pad was removed once the animal started to move. The animal was examined if any excessive bleeding, respiratory arrest or problem with vocalization.The bladder of the animal was manually expressed twice daily. In addition to that, 1 cc of Gentocin (5mg/kg body weight of animal) (Vedco Inc., USA) was administered subcutaneously once a day for 5 consecutive days to prevent gram negative infections [22]. The rat was weighted at day 1, 4, and 7 for the first week. The 0.9% saline was injected subcutaneously according to body weight of the animals. Vitamin C was given to maintain urine acidity[26]. The skin sutures were removed on day 7 post injury.
Open Field Test (Basso, Beattie, Breshnan Locomoting Scale)
All hindlimbs of the rats were assessed by Open Field Locomotor Testing using Basso Beattie Breshnan (BBB) Locomotor Rating Scale [14,15]. The test was performed at day 1, day 4, day 7 and day 14 post injury. The rats were tested for 4 min every 9 am in the morning at one session. All behavioral assessments were performed by two investigators who were blinded to the information of treated groups and shams group. The 21 Point BBB Locomotor Rating Scale was refer to Basso et al., (1996). The rats were recorded using full view of the open field (SHARP Camcoder,JAPAN). The examiners reviewed the tape and re-evaluated the performances to validate the score.
Sacrifice of the animal via perfusion fixation method
After day 14 of treatments, all the rat were deep anaesthesized with lethal dose of Ketamine (120 mg/kg)(Troy Laboratories Pty Limited, Australia) and Xylazine (14 mg/kg) (Troy Laboratories Pty Limited, Australia) intraperitoneally. The lethal dose cocktail mixtures were 1.5 times higher than the surgery anaesthesize dose (Ketamine (80 mg/kg)/Xylazine (10 mg/kg)). The rat was examined for absence of any response to stimulation after 10 minutes after the injection. Following absence of pinch reflex, the rat was restrained in a supine position on the tray. The abdominal skin was cut exposing the chest cavity. The modified 23 G needle was inserted into the apex of the left ventricle. An outlet for the perfusion solution to flow out was created by making a snip over the right atrium. Perfusion was then performed by gravity method using first phosphate-buffered saline (PBS) [27,28] until the clear fluid was flushed out (approximately 120 mL). This is essential to avoid non specific staining by red blood cells (BD Pharmingen Protocols website). This was followed by 500 mL of cold 4% paraformadehyde in phosphate buffer (PB) 0.1 mol/litre (pH = 7.4). The pale color of liver was indicated the perfusion was done. The head and neck also were stiffening after 3 minutes of infusion. The rat was positioned into ventral position to drain the fluid prior to dissection.
Eriochrome Cyanine (EC) staining
Eriochrome Cyanine (EC) staining was adapted from [29].Briefly, the slides were allowed to air dry at room temperature for 10 minutes. The slides were stained in Eriochrome Cyanine (EC) solutions for 30 minutes at room temperature. The EC solutions were filtered before used. After that, the slides were washed briefly in running tap water. Subsequently, the slides were differentiated in 5 % Iron Alum (Sigma, Germany) for 15 minutes at room temperature. Again, the slides were washed briefly in running tap water. After that, the slides were differentiated in the borax-ferricyanide solution (Sigma, Germany) at room temperature for 10 minutes. The slides were then briefly washed in running tap water. Later, the slides were dehydrated through graded ethanol solutions (70 %, 95%, 100%) (HmbG Chemicals, Germany). The slides were cleared through three changes of Xylene. The coverslip were put onto the slides with a permanent mounting medium.
Hematoxylin & Eosin (H&E) staining
The staining procedure was followed [30] with slight modification. Briefly, the slides were allowed to air dry for 10 minutes to completely remove the moisture. The slides were immediately fixed in neutral buffered formalin for 2-5 minutes. The slides were briefly rinsed with running tap water. Later, the slides were stained with filtered 0.1% Mayer Hematoxylin for 10 minutes. The slides then were rinsed in running double distilled water (ddH20) for 5 minutes. After that, the slides were dipped slowly in 0.5% Eosin for 12 times (1.5 g Eosin was dissolved in 300 mL of 95 % ethanol solution (EtOH). The slides were carefully dipped in distilled water (dH20) for 3-5 times. Next, the slides were allowed to dip in 50 % EtOH (10 times), followed by 70 % EtOH for 10 times. Later, the slides were equilibrated in 95 % EtOH for 30 seconds, subsequently in 100 % EtOH for approximately 1 minute. After that the slides were cleared through in three changes of Xylene. The coverslip were put into the slides with permanent mounting medium.
Morphometric Analysis of cross sectioned of spinal cord tissues
Using hematoxylin and eosin staining, the hemorrhage area of lesion was identified microscopically by the presence of distrupted tissue and erythrocytes. The extent of hemorrhage area (mm2) in both white and gray matter was assessed. White and gray matters in injured and treated regions were measured the distribution of hemorrhage regions in each animal. The data was presented as mean ± SEM in all experimental cross sectioned. The sparing of white matter was assessed by Eriochrome cyanide (EC) staining of the cross sectioned of spinal cord. White matter in injured and treated regions was normalized to white matter in non-injured regions in each animal. The data of white matter area in injured regions (WMAi) and white matter area in non-injured regions (WMAn) were presented as mean of area (mm2) ± SEM, whereby white matter sparing was represented as percentages. [(WMAn)-(WMAi)/(WMAn) x 100] for each animal.The calculation of distribution of hemorrhage area and white matter sparring in this study was using Cavalieri’s method of point counting (Ditor et al.,2008).Both of the measurement were using Bioanalyst software with Image Analyser (Olympus XC50-BX41TF, Japan).
Statistical analysis
The One Way ANOVA analysis was performed to validate the contusion injury across experimental groups using New York University (NYU) Impactor device. The improvement of BBB score amongst experimental groups versus time and group were analysed using General Linear Model Repeated Measure ANOVA (GLM). The data was represented by the BBB scores versus time and between groups. The extent and distribution of haemorrhage was analysed by using Mann Whitney Test with bonferroni correction. The values represented by the mean areas (mm2) of hemorrhage in the gray or white matter of the cord ± SEM.The correlation between locomotor activity and distribution of hemorrhage area on gray and white matter after contusion injury were analysed using linear regression.The correlation between locomotor activity and white matter sparring was accomplished using linear regression. All tests were performed using SPSS 18.0.0 program. p<0.05 was considered statistically significant.
Results
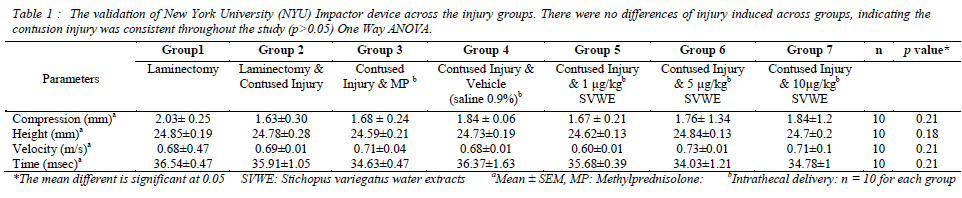
To determine the consistent injury produced by NYU spine impactor across the all experimental groups, we measured the compression rate, heights, velocity and time of impact during the injury. There were no significant differences between the compression rate, height of the impact, velocity of the impact and time course during the impact across all experimental groups (p>0.05)(Table 1).
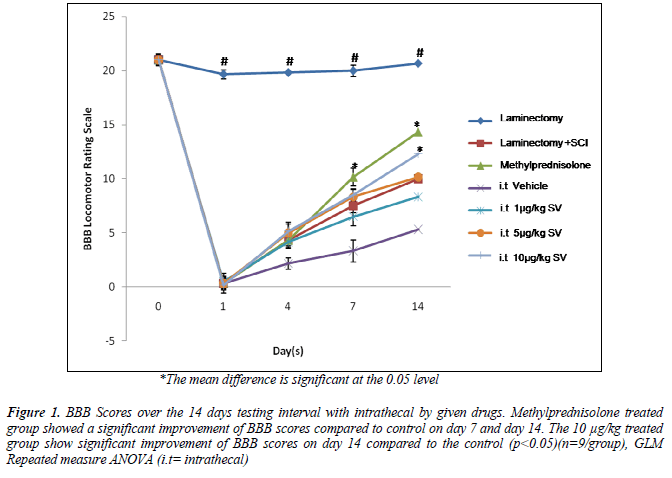
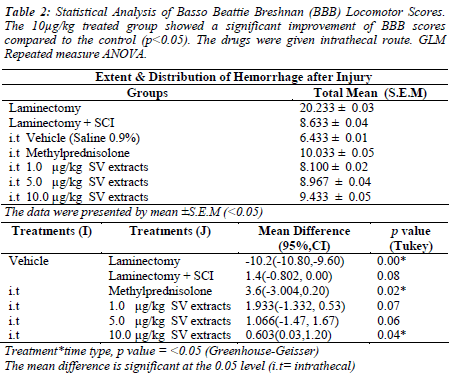
The methylprednisolone and 10μg/kg S.variegatus extracts treated group showed significant improvement of BBB locomotor scores compared to the control group on day 7 and 14 (p<0.05). The progressive improvement was seen from the lack of hindlimb movement (Score 0 on day 1) to a frequent weight support behavior of the spinal injury rat with occasional forelimb and hindlimb coordination (Score 11). Other treated groups showed no significant differences in locomotor scores from day 1 to day 14 (p>0.05)(Figure 1)(Table2).
Figure 1. BBB Scores over the 14 days testing interval with intrathecal by given drugs. Methylprednisolone treated group showed a significant improvement of BBB scores compared to control on day 7 and day 14. The 10 μg/kg treated group show significant improvement of BBB scores on day 14 compared to the control (p<0.05)(n=9/group), GLM Repeated measure ANOVA (i.t= intrathecal)
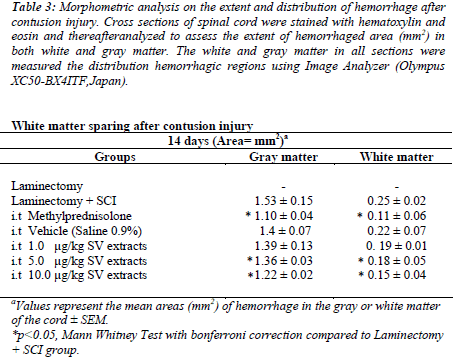
To assess the extent and distribution of injury after a contusive spinal cord injury, the spinal sections were stained with H & E staining. The reddish area in the gray and white matter represented the hemorrhage area.The methylprednisolone and 10μg/kg treated group showed a significant reduction of hemorrhage area in both the white and gray matter of spinal cord tissues compared to the control group (p<0.05) (Table 3).
Table 3: Morphometric analysis on the extent and distribution of hemorrhage after contusion injury. Cross sections of spinal cord were stained with hematoxylin and eosin and thereafteranalyzed to assess the extent of hemorrhaged area (mm2) in both white and gray matter. The white and gray matter in all sections were measured the distribution hemorrhagic regions using Image Analyzer (Olympus XC50-BX4ITF,Japan).
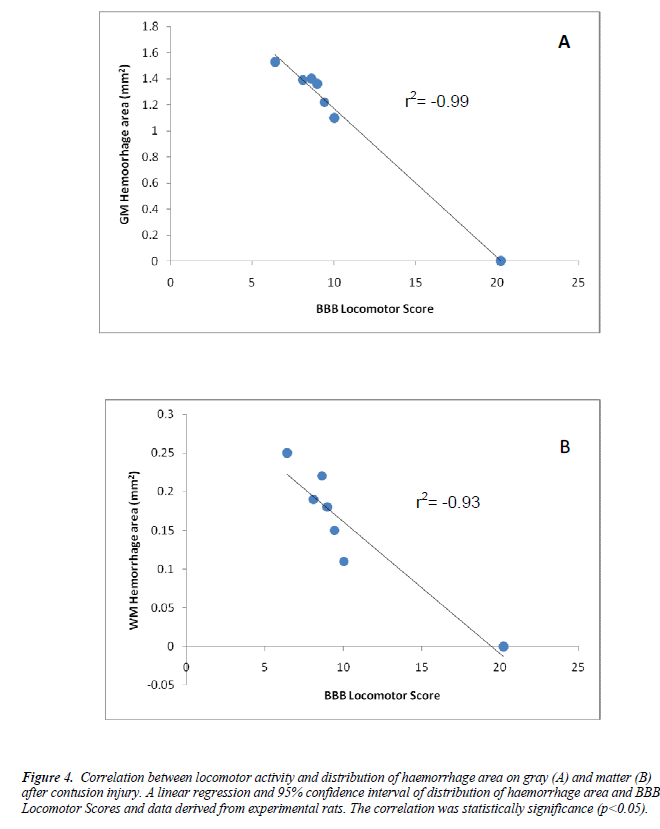
We further evaluated the histopathology assessments on the effect of intrathecal crude extracts on dorsolateral and ventrolateral of experimental spinal cord sections after the contusive spinal cord injury. The methylprednisolone and 10μg/kg treated group show to preserve the neurons after the injury (Figure 2&3).Independent linear regression was performed between the BBB scores and distribution of hemorrhage on both white and gray matter. The BBB test results strongly correlated with gray matter hemorrhage (R=0.99,p<0.05) and white matter (R=0.93, p<0.05) (Figure 4).
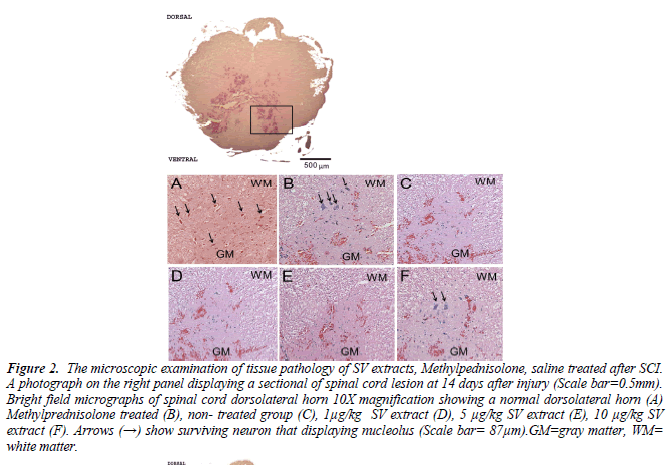
Figure 2. The microscopic examination of tissue pathology of SV extracts, Methylpednisolone, saline treated after SCI. A photograph on the right panel displaying a sectional of spinal cord lesion at 14 days after injury (Scale bar=0.5mm). Bright field micrographs of spinal cord dorsolateral horn 10X magnification showing a normal dorsolateral horn (A) Methylprednisolone treated (B), non- treated group (C), 1μg/kg SV extract (D), 5 μg/kg SV extract (E), 10 μg/kg SV extract (F). Arrows (→) show surviving neuron that displaying nucleolus (Scale bar= 87μm).GM=gray matter, WM= white matter.
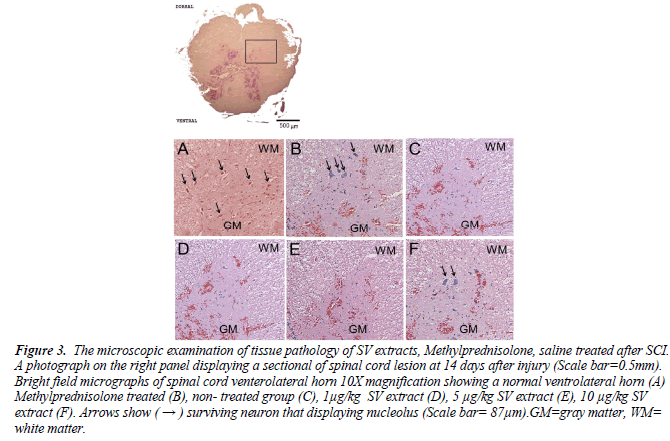
Figure 3. The microscopic examination of tissue pathology of SV extracts, Methylprednisolone, saline treated after SCI. A photograph on the right panel displaying a sectional of spinal cord lesion at 14 days after injury (Scale bar=0.5mm). Bright field micrographs of spinal cord venterolateral horn 10X magnification showing a normal ventrolateral horn (A) Methylprednisolone treated (B), non- treated group (C), 1μg/kg SV extract (D), 5 μg/kg SV extract (E), 10 μg/kg SV extract (F). Arrows show ( → ) surviving neuron that displaying nucleolus (Scale bar= 87μm).GM=gray matter, WM= white matter.
Figure 4. Correlation between locomotor activity and distribution of haemorrhage area on gray (A) and matter (B) after contusion injury. A linear regression and 95% confidence interval of distribution of haemorrhage area and BBB Locomotor Scores and data derived from experimental rats. The correlation was statistically significance (p<0.05).
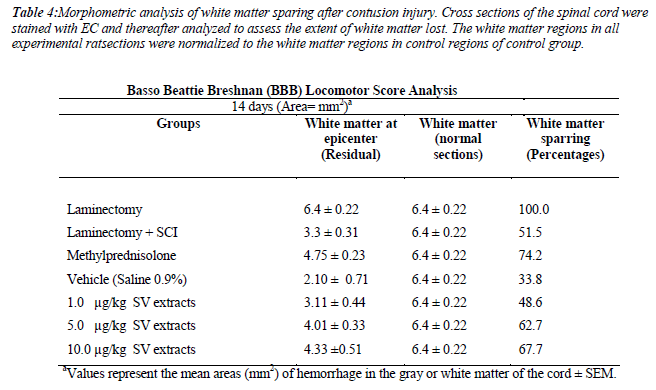
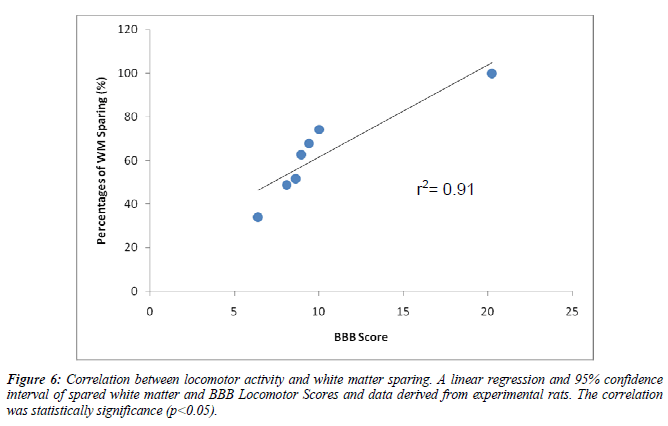
We also measure the white matter sparing after contusion injury. The methylprednisolone(74.2%) and 5μg/kg (62.7%) and 10μg/kg (67.7%) treated group show significance spared the white matter after the contusion injury compare to vehicle group (p<0.05). The BBB test strongly correlated with white matter sparring (R=0.91,p<0.05)(Table 4)(Figure 5, 6).
Table 4: Morphometric analysis of white matter sparing after contusion injury. Cross sections of the spinal cord were stained with EC and thereafter analyzed to assess the extent of white matter lost. The white matter regions in all experimental ratsections were normalized to the white matter regions in control regions of control group.
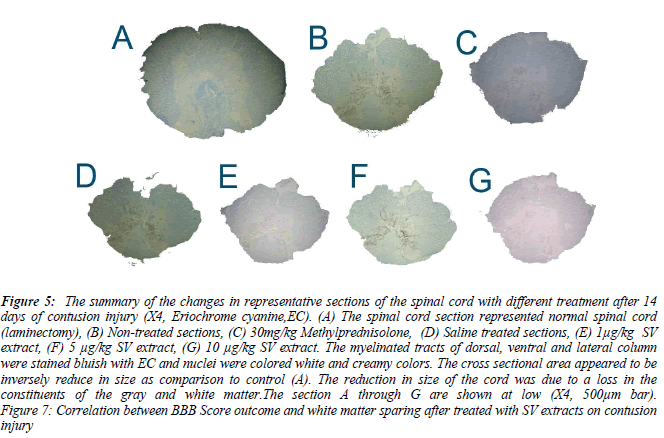
Figure 5: The summary of the changes in representative sections of the spinal cord with different treatment after 14 days of contusion injury (X4, Eriochrome cyanine,EC). (A) The spinal cord section represented normal spinal cord (laminectomy), (B) Non-treated sections, (C) 30mg/kg Methylprednisolone, (D) Saline treated sections, (E) 1μg/kg SV extract, (F) 5 μg/kg SV extract, (G) 10 μg/kg SV extract. The myelinated tracts of dorsal, ventral and lateral column were stained bluish with EC and nuclei were colored white and creamy colors. The cross sectional area appeared to be inversely reduce in size as comparison to control (A). The reduction in size of the cord was due to a loss in the constituents of the gray and white matter.The section A through G are shown at low (X4, 500μm bar). Figure 7: Correlation between BBB Score outcome and white matter sparing after treated with SV extracts on contusion injury
Discussions
We examined the effect of different intrathecal doses (1μg/kg, 5μg/kg and 10μg/kg) of S.variegatus extracts on behavior performances of spinal injury rats. Paraplegia occurred in all the experimental animals at day 0, which is clearly indicated by a score of zero (0). However, most of the spinal injury rats showed occasional reflex spasms when the hind limbs were touched. This movement was not considered when scoring to avoid false positive data. In the present study, the methylprednisolone treated group showed a significant improvement 10 μg/kg treated group of S.variegatus extracts showed a significant improvement to the BBB score (Score 13) at day 14 compared to the control group (p=0.04) but the other treated groups showed no differences compared to the control group (p>0.05). This implies that the S.variegatus extracts demonstrated dose dependent effects. The improvement was seen from the lack of hind limb movement to a frequent weight support behavior of the spinal injury rat with occasional forelimb and hind limb coordination. Furthermore, Jones and Tuszynski (2001) signified that intrathecal infusion is most efficient at 7 days following spinal cord injury but was markedly reduced after 14 days due to occlusion of catheters over time [31].
We used Methylprednisolone (MP) as a positive control drug. MP (200 μg) which was given intrathecally showed a significant improved BBB score starting at day 7 compared to control group (p<0.05). This finding was further supported by Wu et al.,(2007) showed the intrathecal delivery of MP improved behavioral score after 48 hours. The mechanism of MP in neuroprotection may explain the improvement of both of the hindlimb score on BBB rating scale[32]. The corticosteroids can suppress local inflammatory responses that produce edema and swelling and worsen neuronal injuries via the inhibition of pathological stress, induce the release of proinflammatory mediators (prostaglandin, interleukin-1ß, tumor necrosis factor α and nitric oxide)[33,34]. Suberviola et al., (2007) further supported our data that intrathecal MP was maintained the blood spinal barrier, reducing vasogenic oedema and able to stabilize membrane structure led to improvement in motor performances in spinal injury rats.
This study was examined the histopathology of spinal cord tissues after the contused SCI using Hematoxylin and Eosin (H & E) and Eriochrome Cyanine (EC) staining. The experimental spinal cord tissues later were analyzed using morphometric techniques in order to characterize and compare the treatments given after contused SCI. The major findings of our current study were (1) intramedullary hemorrhage was more prominent in gray than white matter. (2) The area of hemorrhage were observed highly negative correlation between gray matter (r2=0.99) rather than white matter hemorrhage (r2=0.93)(p<0.001) at day 14 of contusion injury. (3) There were remaining Ventral Motor Neurons (VMNs) after contused spinal injuries on day 14, indicating that there were spared VMNs after treatment of SV extracts and Methylprednisolone treated groups.
The spinal tissues treated with Methylprednisolone and 10 μg/kg of SV extracts were showed reduction in hemorrhage area (mm2) on gray and white matter compared to injury group without any treatment given (p<0.05). Our study showed the intramedullary hemorrhage was prominent in gray matter (1.4 ± 0.15 mm2) than white matter (0.22 ± 0.02 mm2) in injury group(p<0.05). This findings which have been previously reported by [35, 36] indicating that the disruptions of blood supply to spinal cord via central gray matter. The distributions of hemorrhage area, however, were found extended to white matter of spinal cord in all experimental spinal tissues after 14 days. Therefore, we suggested that 10μg/kg of SV extract could reduce the internal bleeding (hemorrhage) in spinal contusion injury. This may explaining the improvement of the BBB Locomotor scale of a 10μg/kg of SV extracts and methylprednisolone treated injured rats.
To further understand the relationship between BBB score and distribution of hemorrhage area, the correlation coefficient analysis was made. We found both gray matter (r2=0.99) and white matter hemorrhage (r2=0.93) were showed highly negative correlation to BBB score at day 14 of contusion injury in all experimental tissues (p<0.001). The low scores on BBB Locomotor scale indicated the severity of hemorrhage.
The amount of residual white matter after the thoracic injury (T9-T10) spinal injury has been shown to correlate with behavioral recovery after a spinal cord injury[37]. In the present study, the residual white matter after the injury and treatment were analyzed. Interestingly, the Methylprednisolone treated group showed significant differences in sparing white matter (74.2%) compared to the injury group. In addition, the SV extracts at the dose of 10μg/kg (67.7%) and 5 μg/kg (62.7%) also showed a significance of white matter sparing compared to the injury group. We further examined the relationship between white matter sparing and functional outcome (final BBB score). Our data indicated that a significant correlation was found between the final BBB functional outcome and white matter sparing (r2=0.91). A relationship between white matter sparring and functional outcome after a spinal cord injury relies upon the assumptions that damage to myelinated fiber tracts will be affected in functional recovery [38,39]. The low score of BBB amongst experimental rats was suggested to be due to the disruptions of ascending and descending axonal transmission to lower extremities.
We examined Ventral Motor Neurons (VMNs) that were responsible for motor response at the ventral horns. The VMNs were found to be reduced in all treated groups compared to control after day 14 of the injury (p<0.05). The reduction in VMNs was probably due to tissue lost over time most probably due to necrosis and apoptosis. We found that the remaining intact VMNs in a 10 μg/kg of the S.variegatus extract treated group as well as the methylprednisolone treated group after the spinal cord injury. This suggests that the surviving VMNs were involved in re-myelination process which explained the improvement of BBB scores amongst Methylprednisolone and 10 μg/kg treated groups. The reduction of VMNs lost indicated that VMNs may be vulnerable to the common factor in the secondary injury cascade [40].
In conclusion, our results demonstrated that the Malaysian SVWE is able to promote functional recovery after acute spinal cord injury and reduces intramedullary hemorrhage. However, methylprednisolone showed a superior treatment towards acute spinal cord injury. Further studies need to be carried out to strengthen the present hypothesis.
Acknowledgement
This study was supported by Short Term Grant from Universiti Sains Malaysia (304/PPSP/6150068).
References
- Ridzwan BH, Kaswandi MA, Azman Y, Fuad M: Screening for antibacterial agents in three species of sea cucumbers from coastal areas of Sabah. General Pharmacology: The Vascular System 1995, 26(7): 1539-1543.
- Ridzwan BH: Sea Cucumber : A Malaysian Heritage, vol. 1, 1st edn. Kuala Lumpur: Research Centre International Islamic University Malaysia; 2007.
- Ridzwan BH, Zarina MZ, Kaswandi MA, Nadirah M, Shamsuddin AF: The antinociceptive effects of extracts from Stichopus chloronatus Brandt. Pakistan Journal of Biological Sciences 2001, 4(3): 244-246.
- Idid SZ, Azidah AA, Ridzwan BH: The effect of coelomic fluid from Stichopus variegatus Semper given intraperitoneally upon the turnover rate of the brain amines in adult rats chronically induced with alcoholic liver damage. Kuala Lumpur: UKM; 2003.
- Tan WT, Fong YS, Ridzwan BH: The Effect of Coelomic Fluid Stichopus Hermanii on Isolated Perfused Rat Hearts and Involvement of Prostaglandin in its Mechanism of Action. Pakistan Journal of Biological Sciences 2005, 8(1): 78-84.
- Hawa I, Zulaikah M, Jamaludin M, Zainal Abidin AA, Kaswandi MA, Ridzwan BH: The potential of the coelemic fluid in sea cucumber as an antioxidant. Mal J Nutr 1999(5):55-56.
- Tong Y, Zhang X, Tian F, Yi Y, Xu Q, Li L, Tong L, Lin L, Ding J: Philinopside A, a novel marine-derived compound possessing dual anti-angiogenic and antitumor effects. Int J Cancer 2005, 114:843-853.
- Azim, P., Mohsin, SSJ., Hasnan, J., Abdullah, J. Analysis of Sea Cucumber Body Wall Extracts From Perhentian Stichopus variegatus species. Eur J of Scientific Res 2012,68(1): 54-57.
- Minamiguchi K, Keikot K, Nagase H, Sasaki K, Ohwada K, Kenji K: Depolimerized Holothurian Glycoaminoglycans (DHG), a novel alternative anticoagulant for hemodialysis is safe and effective in a dog renal failure. Kidney International 2004, 63(4):1548-1555.
- Zhang Y, Song S, Liang H, Wang Y, Wang W, Ji A: Enhancing effect of a sea cucumber Stichopus japonicus sulfated polysaccharide on neurosphere formation in vitro. Journal of Bioscience and Bioengineering 2010, 110(4):479-486.
- Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR: Pathophysiology and pharmacologic treatment of acute spinal cord injury. 2004, 4:451-464.
- Taoka Y, Okajima K: Spinal cord injury in the rat. Progress in Neurobiology 1998, 56:341-358.
- Young W: Secondary injury mechanisms in acute spinal cord injury. J Emerg Med 1993, 11:13-22.
- Basso DM, Beattie MS, Bresnahan JC: A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995, 12(1):1-21.
- Basso DM, Beattie MS, Bresnahan JC: Graded Histological and Locomotor Outcomes after Spinal Cord Contusion Using the NYU Weight-Drop Device versus Transection. Experimental Neurology 1996, 139:244-256.
- Furuta M, Suwa T, Kuwabara Y, Otsuhata K, Takeda A: Electron-beam sterilization of laboratory animal diets--sterilizing effect of 10-MeV electrons from a linear accelerator. Exp Anim 2002, 51(4):327-334.
- Medinaceli LD: An Anatomical Landmark for Procedures on Rat Thoracic Spinal Cord. Experimental Neurology 1986:404-408.
- Hook MA, Liu GT, Washburn SN, Ferguson AR, Bopp AC, Huie JR, Grau JW: The impact of morphine after a spinal cord injury. Behaviour Brain Research 2007, 179:281-293.
- Casas CE, Herrera LP, Prusmack C, Ruenes G, Marcillo A, Guest JD: Effects of epidural hypothermic saline infusion on locomotor outcome and tissue preservation after moderate thoracic spinal cord contusion in rats. J Neurosurg Spine 2005, 2(3):308- 318.
- Vialle LRG, Grochocki LR, Nohama P: NYU device: automation of the Weight-drop rod. In: World Congress of Medical Physics and Biomedical Engineering. Springer Berlin Heidelberg; 2006: 728-730.
- Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF et al: Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci 1997; 17(14): 5395-5406.
- Baker KA, Nakashima S, Hagg T: Dorsal column sensory axons lack TrkC and are not rescued by local neurotrophin-3 infusions following spinal cord contusion in adult rats. Exp Neurol 2007; 205(1):82-91.
- Lee JK, Johnson CS, Wrathall JR: Up-regulation of 5- HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp Neurol 2007; 203(2): 502-511.
- Abe C, Tashiro T, Tanaka K, Ogihara R, Morita H: A novel type of implantable and programmable infusion pump for small laboratory animals. J Pharmacol Toxicol Methods 2009; 59(1): 7-12.
- Choi JS, Kim HY, Cha JH, Choi JY, Chun MH, Lee MY: Upregulation of vascular endothelial growth factor receptors Flt-1 and Flk-1 in rat hippocampus after transient forebrain ischemia. J Neurotrauma 2007; 24(3): 521-531.
- Lindsey AE, LoVerso RL, Tovar CA, Hill CE, Beattie MS, Bresnahan JC: An analysis of changes in sensory thresholds to mild tactile and cold stimuli after experimental spinal cord injury in the rat. Neurorehabil Neural Repair 2000; 14(4): 287-300.
- Adickes ED, Folkerth RD, Sims KL: Use of perfusion fixation for improved neuropathologic examination. Arch Pathol Lab Med 1997; 121(11): 1199-1206.
- Tenkova TI, Goldberg MP: A Modified Silver Technique (de Olmos Stain) for Assessment of Neuronal and Axonal Degeneration. In: Neuroprotection methods and protocols. vol. 399. London: Humana Press; 2007: 31-39.
- Leung PY, Wrathall JR: Local and distal responses to injury in the rapid functional recovery from spinal cord contusion in rat pups. Exp Neurol 2006; 202(1): 225- 237.
- Fukunaga S, Sasaki S, Fu T, Yokoyama H, Lee I, Nakagaki I, Hori S, Tateishi H, Maruo S: Experimental study of neural repair of the transected spinal cord using peripheral nerve graft. J Orthop Sci 2004; 9(6): 605-612.
- Jones, Tuszynski M: Chronic intrathecal infusions after spinal cord injury cause scarring and compression. Microsc Res Techol 2001; 54: 317-324.
- Wu G-J, Chen W-F, Sung C-S, Jean Y-H, Shih C-M, Shyu C-Y, Wen Z-H: Preventive Effects Of Intrathecal Methylprednisolone Administration On Spinal Cord Ischemia In Rats: The Role Of Excitatory Amino Acid Metabolizing Systems. Neuroscience 2007; 147: 294- 303.
- Rhodes KE, Moon LD, Fawcett JW: Inhibiting cell proliferation during formation of the glial scar: effects on axon regeneration in the CNS. Neuroscience 2003; 120(1): 41-56.
- Popovich PG, Guan Z, McGaughy V, Fisher L, Hickey WF, Basso DM: The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J Neuropathol Exp Neurol 2002; 61(7): 623- 633.
- Allen A: Surgery of the experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. J Am Med Assoc 1911; 57: 878-890.
- Assenmacher P, Ducker T: Experimental traumatic paraplegia. The vascular and pathological changes seen in reversible and irreversible spinal cord lesions. J Bone Joint Surg 1971; 53A: 671-680.
- Taoka Y, Okaijima K: Spinal cord injury in rats. Progress in Neurobiology 1998; 56: 341-358.
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG: Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 2006; 23(5): 635-659.
- Ma M, Wei P, Wei T, Ransohoff RM, Jakeman LB: Enhanced axonal growth into a spinal cord contusion injury site in a strain of mouse (129X1/SvJ) with a diminished inflammatory response. J Comp Neurol 2004; 474(4): 469-486.
- Grossman SD, Rosenberg LJ, Wrathall JR: Temporalspatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol 2001; 168(2): 273- 282.