Review Article - Biomedical Research (2017) Volume 28, Issue 18
The association of diabetes mellitus and gender with risk of bone fractures: A systematic review and meta-analysis
Mansour Amraei1#, Shahram Mohamadpour2, Yaser Seifinejad3, Seyedeh Fatemeh Mousavi4, Ehsan Shirzadpour3#, Safoura Mohamadpour4 and Ardeshir Moayeri2*
1Department of Clinical Physiology, Faculty of Medicine, Ilam University of Medical Sciences, Ilam, Iran
2Department of Clinical Anatomy, Faculty of Medicine, Ilam University of Medical Sciences, Ilam, Iran
3Department of Clinical Biochemistry, Faculty of Medicine, Ilam University of Medical Sciences, Ilam, Iran
4Prevention of Psychosocial Injuries Research Center, Ilam University of Medical Sciences, Ilam, Iran
#These authors have equally contributed to this study.
- *Corresponding Author:
- Ardeshir Moayeri
Department of Clinical Anatomy
Ilam University of Medical Sciences, Iran
Accepted on August 03, 2017
Abstract
Bone fracture shows a growing trend. This increase is a result of several factors occurs. In this study, a meta-analysis was performed to examine the relationship of fracture risk with Type 2 diabetes mellitus and gender. For this purpose, we searched the credible databases including PubMed, ISI, and Scopus for the related epidemiologic studies that were published up to May 2016. The data provided in these articles was extracted and then analyzed by a random-effects model. Funnel plot was used to check for publication bias. Statistical analyses were carried out by R version 3.2.1 and STATA (version 11.1). After filtering irrelevant articles, 30 studies were selected for meta-analysis. The analyses showed that type 2 diabetes mellitus is positively associated with overall fracture risk (summary RR=1.05, 95% CI: 1.04, 1.06). The incidence of fractures was higher in men than in women (Phet=0.043). The interpretation of the meta-regression showed that no significant relationship exists between the gender risk factor in diabetic patients and years of study (P=0.280). The results highlight the importance of adopting suitable strategies for prevention of bone fracture in T2DM patients.
Keywords
Diabetes mellitus, Bone, Osteoporosis, Fractures, Meta-analysis.
Introduction
Type 2 Diabetes Mellitus (T2DM) is a metabolic disorder appertaining to hyperglycemia associated with insulin secretion and resistance disorders [1,2]. Patients with Type 2 diabetes mellitus often suffer from not only direct micro and macrovascular complications, but also indirect skeletal disorders, such as fragile bones and frequent bone fractures [3]. The latter effect is the result of multiple and sometimes contradictory associations between diabetes and bone quality as well as possible impact of diabetes on bone turnover [4,5].
Research has shown that patients with Type 2 diabetes mellitus have a normal to elevated Bone Mineral Density (BMD) and because of this association, there used to be a common misconception that these patients are safe against osteoporosis [6]. But later, it became clear that in T2DM patients, BMD is only one of several fracture risk factors, and despite their elevated BMD, these patients have a higher risk of bone fracture [7,8]. Thus, diabetes may be associated with a reduction of bone strength and develop bone fragility that is, not reflected in the measurement of BMD [6].
Further studies in this regard showed that how diabetes affects the fracture risk depends on gender and age of patient, history of decease and medication, and the location of fracture, [6,9-11]. Research has shown that fracture risk is directly associated with disease duration, the presence of diabetic complications, inadequate glycemic control, and insulin use [12].
The association of T2DM with fracture risk has been extensively researched but the results have been largely contradictory, as different studies have reported positive relationship, inverse relationship, and sometimes no relationship between T2DM and fracture risk [3-20]. Since a meta-analysis is warranted to clarify the association between T2DM and fracture risk, the present study provided a systematic review and meta-analysis of association between type 2 diabetes mellitus and risk of fracture.
Materials and Methods
Search strategy
In this study, the credible databases including PubMed/ Medline, Scopus, and ISI Web of Knowledge were searched for all articles with keywords “diabetes mellitus” , “type 2 diabetes mellitus”, “glucose”, “insulin”, “fracture”, ‘‘bone”, “osteoporosis”, “bone mineral density” and “risk factors”, in the time frame of 1980 to May 2016. The search was narrowed down to the articles that contained the aforementioned keywords in their title or abstract. The references of the found articles were also reviewed in search for other articles. The list was also narrowed down to human researches. To decrease the bias, all steps of search, elimination of irrelevant papers, and extraction of data from articles were performed by two researchers working independently.
Inclusion and exclusion criteria
All epidemiologic researches on the relationship of T2DM with the risk of fracture in hip (low-trauma), distal forearm, proximal humerus, ankle, and foot, and non-vertebral or vertebral fractures, were included in the study. After compiling a secondary list, we excluded all studies on individuals with T1DM (Type 1 Diabetes Mellitus) or IGT (Impaired Glucose Tolerance), the studies where data required for analysis of standard errors for effect estimates was not provided, all metaanalyses and systematic reviews, the studies with duplicate citation, the studies with parallel reports for the same population or cohort, and dated studies for which we found a recent counterpart.
Data extraction
After finalizing the compilation of studies, we extracted, from every study, the name of the first author, year of publication, country, study design, sample size, gender of subjects, number of fractures, fracture risk estimations and their confidence intervals, factors controlled for by matching or multivariable analysis and adjustment for potential confounders. This data extraction was performed in line with a standard protocol by two researchers working independently, who reviewed the abstracts as well as full articles. Afterwards, a meeting was held and differences between the findings of two researchers were discussed and resolved. The findings were organized into data collection forms and then imported into Microsoft Excel.
Data synthesis and analysis
Meta-analysis was performed using the logarithm of the relative risk and the corresponding standard error. The relative risk estimates and their 95% confidence intervals were acquired by the method of Der Simonian and Laird.
Heterogeneity of the studies was evaluated by the Cochran’s Q, meta-regression and I2. Since studies were highly heterogeneous, we used the random effects model. In search for the sources of heterogeneity, a meta-regression analysis was performed. In this analysis, gender, duration of follow-up, and duration of decease were used as independent variables and the log relative risk was considered as the dependent variable. Publication bias was evaluated with the help of funnel plots and Egger test. A pre-specified sensitivity analysis was also conducted. Statistical analyses were performed using statistical two-sided tests in R Software 3.2.1 and STATA 11.1 with P<0.05 considered as the level of significance (in heterogeneity tests).
Results
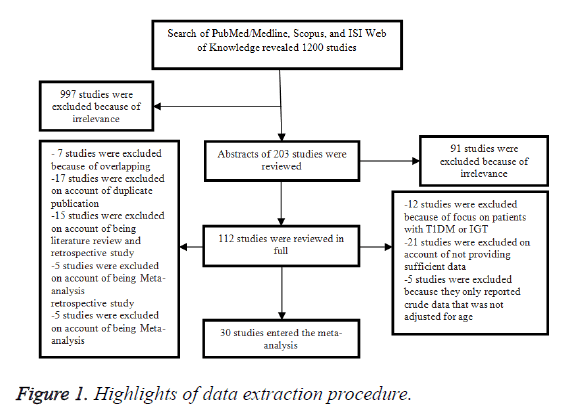
In the initial search, we found about 1200 articles published between 1980 and 2016, but after narrowing down the list by eliminating the irrelevant works, this number was reduced to 203. Of these 203 articles, 91 were eliminated after review of abstract, and the remaining 112 articles were reviewed in full. In this full-text review, we found 30 epidemiologic studies (2 case-control and 28 cohort studies) on the relationship of T2DM and fracture risk (Figure 1) [10,11,20-47]. Table 1 shows the highlights of data extraction procedure.
| Reference | Design | Country | Average follow-up period (y) | Age (y) At enrolment |
N | |
|---|---|---|---|---|---|---|
| N T2DM | N NDM | |||||
| Heath [19] | Cohort | United States | Unknown | <103 | T: 986 | T: 986 |
| Meyer [21] | Cohort | Norway | 10.9 | 35-49 | M:180 F:118 |
M:24333 F:23850 |
| Forsen [22] | Cohort | Norway | 9 | ≥ 50 | M:220 F:274 |
M:13210 F:13685 |
| Nicodemus [10] | Cohort | United States | 11 | 55-69 | F: 1682 | F: 30377 |
| Ivers [11] | Cohort | Australia | 5 | ≥ 49 | T:216 | T: 3438 |
| Ottenbacher [23] | Cohort | United States | 7 | ≥ 65 | T: 690 | T: 2194 |
| de Liefde [24] | Cohort | Netherlands | 6.3 ± 2.3 | ≥ 55 | T: 792 M: 309 F: 438 |
T: 5863 M: 2382 F: 3481 |
| Gerdhem [25] | Cohort | Sweden | 4.6 | 75 | F: 74 | F: 1058 |
| Strotmeyer [26] | Cohort | United States | 4.5 | 70-79 | T: 566 | T: 2236 |
| Bonds [27] | Cohort | United States | 7 | 64.9 ± 7 | F: 5285 | F: 88120 |
| Holmberg [28] | Cohort | Sweden | 17 | 27-68 | M: 276 F: 166 |
M: 22444 F: 10902 |
| Dobnig [29] | Cohort | Australia | 2 | ≥ 70 | F: 583 | F: 1081 |
| Ahmed [30] | Cohort | Norway | 6 | 25-98 | M: 175 F: 198 |
M: 12639 F: 14065 |
| Janghorbani [31] | Cohort | United States | 20.4 ± 3.4 | 34-59 | F: 8345 | F: 101343 |
| Leslie [32] | Cohort | Canada | 10 | ≥ 20 | T: 82,094 42,874 newly diagnosed, 16,081 short duration, 23,139 long duration | T: 236682 |
| Lipscombe [33] | Cohort | Canada | 6.1 | ≥ 66 | M:100322 F: 97090 |
M:202875 F:198525 |
| Melton [34] | Cohort | United States | 11.8 | 30-97 | T:1964 M: 992 F: 972 |
- |
| Chen [35] | Cohort | Taiwan | 6 | ≥ 35 | M:227289 F:257498 |
M:227303 F:257303 |
| Mancini [36] | Cohort | Italy | 13 | 44-82 | M: 43 | M: 22 |
| Yamamoto [37] | Cohort | Japan | - | ≥ 50 | M: 161 F:137 |
M: 76 F: 622 |
| Schneider [38] | Cohort | United States | 20 | 45-64 | T: 1195 M: 501 F: 694 |
T: 13340 M: 5936 F: 7404 |
| Chung [39] | Cohort | Korea | - | ≥ 50 | F: 2239 | - |
| Oei [40] | Cohort | Netherlands | 12.2 | ≥ 55 | T: 217 | T: 3715 |
| Reyes [41] | Cohort | Spain | 2.55 | ≥ 65 | M: 36865 | M: 149306 |
| Napoli [42] | Cohort | United States | 9.1 | ≥ 65 | M: 801 | M: 3086 |
| Leslie [43] | Cohort | Canada | 6 | ≥ 40 | T: 6455 | T: 55958 |
| Hothersall [44] | Cohort | Scotland | 2 | 20-84 | T: 180841 | T: 3066000 |
| Looker [45] | Cohort | United States | 6.7 | ≥ 65 | NHW: 398 NHB: 178 MA: 226 |
NHW 2554 NHB: 631 MA: 601 |
| Kegan [46] | Case- control | United States | - | ≥ 45 | T: 1913 | T: 1913 |
| Vestergaard [47] | Case- control | Denmark | - | 43 ± 27 | T: 3241 | T: 6375 |
Table 1. Fracture risk in diabetic patients compared to healthy subjects.
The primary meta-analysis was performed on all 30 studies found in the literature, which included 2 case-control and 28 cohort studies. After finding that reported prevalence is highly heterogeneous (P<0.001), it was decided that meta-analysis should be performed through random effect method.
We included 30 studies in the primary meta-analysis (Figure 1).
Characteristics reviewed articles about associated diabetes mellitus and risk of bone fractures is presented in Table 1.
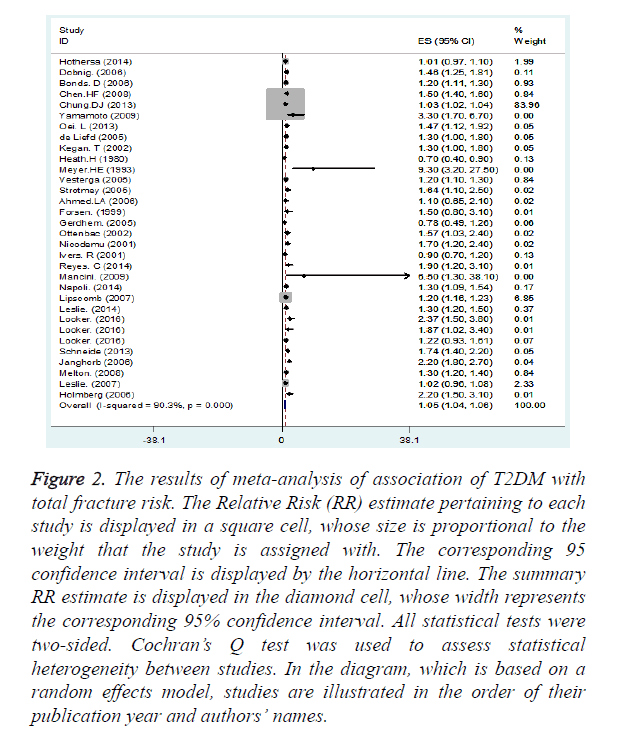
Figure 2 shows a summary of the results of studies on the relationship of T2DM with the total sum of fracture incidence. As shown in this figure, a statistically significant positive relationship was found between T2DM and total fracture incidence (summary RR=1.05, 95% CI: 1.04, 1.06).
Figure 2. The results of meta-analysis of association of T2DM with total fracture risk. The Relative Risk (RR) estimate pertaining to each study is displayed in a square cell, whose size is proportional to the weight that the study is assigned with. The corresponding 95 confidence interval is displayed by the horizontal line. The summary RR estimate is displayed in the diamond cell, whose width represents the corresponding 95% confidence interval. All statistical tests were two-sided. Cochran’s Q test was used to assess statistical heterogeneity between studies. In the diagram, which is based on a random effects model, studies are illustrated in the order of their publication year and authors’ names.
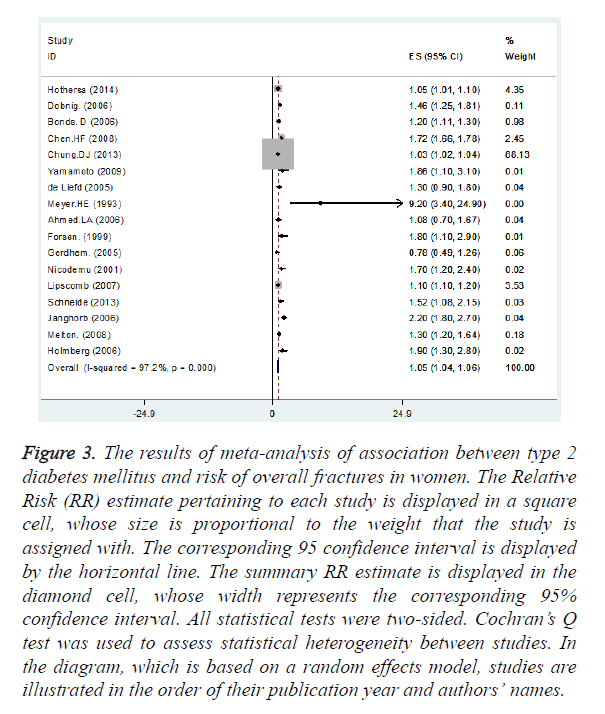
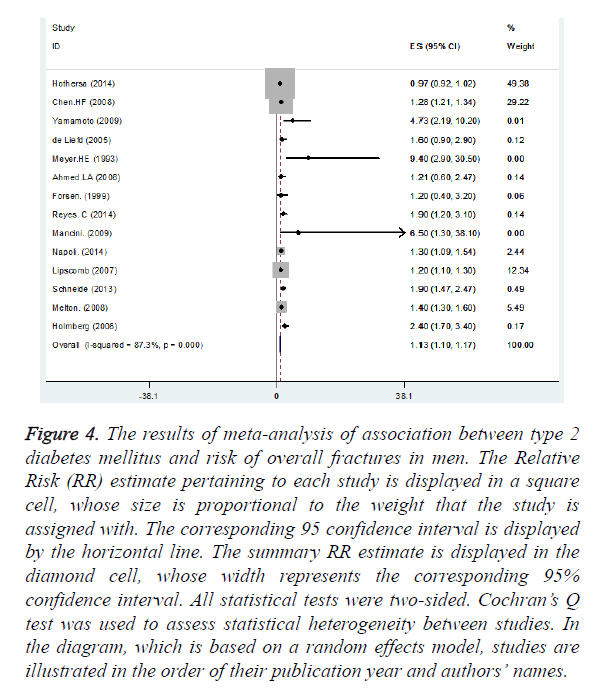
Nine and thirteen studies had reported the association between T2DM and fracture incidence in female and male, respectively. We found statistically significant positive association between T2DM and overall fracture incidence in female (summary RR=1.05, 95% CI: 1.04, 1.06) (Figure 3) and in male (summary RR=1.13, 95% CI: 1.10, 1.17) (Figure 4).
Figure 3. The results of meta-analysis of association between type 2 diabetes mellitus and risk of overall fractures in women. The Relative Risk (RR) estimate pertaining to each study is displayed in a square cell, whose size is proportional to the weight that the study is assigned with. The corresponding 95 confidence interval is displayed by the horizontal line. The summary RR estimate is displayed in the diamond cell, whose width represents the corresponding 95% confidence interval. All statistical tests were two-sided. Cochran’s Q test was used to assess statistical heterogeneity between studies. In the diagram, which is based on a random effects model, studies are illustrated in the order of their publication year and authors’ names.
Figure 4. The results of meta-analysis of association between type 2 diabetes mellitus and risk of overall fractures in men. The Relative Risk (RR) estimate pertaining to each study is displayed in a square cell, whose size is proportional to the weight that the study is assigned with. The corresponding 95 confidence interval is displayed by the horizontal line. The summary RR estimate is displayed in the diamond cell, whose width represents the corresponding 95% confidence interval. All statistical tests were two-sided. Cochran’s Q test was used to assess statistical heterogeneity between studies. In the diagram, which is based on a random effects model, studies are illustrated in the order of their publication year and authors’ names.
The results of meta-analysis of association of T2DM with fracture risk by sex showed although there was a statistically significant positive association between T2DM and overall fracture incidence in both men and women; the estimation of summary was stronger in men than in women (Phet=0.043) (Table 2).
| Subgroup | No. of studies |
Summary relative risk |
0.95 confidence interval | Between studies | Between subgroups | ||
|---|---|---|---|---|---|---|---|
| I2 | p for heterogeneity |
I2 | p for heterogeneity | ||||
| Sex | |||||||
| Female | 7 | 1.44 | 1.18-1.70 | 0.913 | 0 | 0.913 | 0.043 |
| Male | 3 | 1.9 | 1.3- 2.58 | 0 | 0 | ||
| Both | 23 | 1.17 | 1.15-1.19 | 0.854 | 0 | ||
Table 2. Gender risk factors for the association between type 2 diabetes and fracture risk.
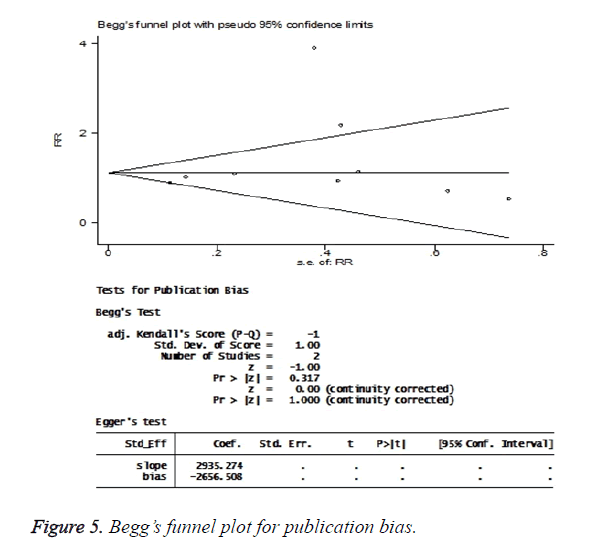
The publication bias tests found no significant bias effect in these studies. In the Begg’s regression asymmetry test, P value was found to be 0.32 (Figure 5).
Meta-regression analysis found no significant relationship between the diabetic patients’ gender risk factor and the year of study (P=0.280).
Discussion
The results of this study provide an overview of the relationship of T2DM and fracture risk, which are two important medical conditions with social and economic consequences for the patients and society. Previous studies have given inconsistent results in regard to the relationship of diabetes and overall risk of fracture, as some have reported significant positive association between these two while others have found no such relationship [11,25,26,28,34,38,40,43,47].
The present study suggests that there is indeed a positive relationship between T2DM and total fracture risk (summary RR=1.17, 95% CI: 1.15, 1.20), which supports the reports of previous meta-analyses on this particular subject [6,9]. Sex specific differences may exist, reflecting differential patterns of bone apposition between sexes (bone dimorphism) [48]. Also there might be gender differences in bone metabolism, which could be due to differences in body composition and differences in sex hormones (e.g. postmenopausal status in most studied women) [2]. A direct interaction between estradiol and IGF-I in the determination of periosteal apposition has been proposed, and serum IGF-I levels are negatively associated with increased risk for prevalent fractures in postmenopausal women but not in men with type 2 diabetes [19,48-50].
In the present study, it was found that diabetic women have a lower overall risk of fracture than diabetic men (Phet=0.043).
There are reports suggesting the presence of hypogonadism in 21-64% of male T2DM patients especially among elderly, so diabetes-induced hypogonadism can be counted among the risk factors of secondary osteoporosis. From another perspective, it has been shown that smoking, alcohol consumption, anticonvulsant treatment, physical inactivity, and low free androgen index significantly increase the relative risk of fracture in men [51-54].
The results of this study point to the significant effect of T2DM on the public health impact of fractures. The limitations of this meta-analysis included the inability to assess the effect of ethnicity because of lack of sufficient data about such association in different ethnicities, and also the lack of sufficient BMD data that may contribute to the observed relationships, which makes it impossible to control the impact of BMD on this relationship.
There was also the incidence of fractures increased in T2DM men than in T2DM women. Overall, the results of this metaanalysis strongly support an association between type 2 diabetes and increased risk of any fracture. With a worldwide increasing prevalence of diabetes, the contribution of diabetes to the incidence of low-trauma fracture may increase.
The results of this study provide an overview of the relationship of T2DM and overall fracture risk and highlight the need for new risk prediction models that would be able to account for the risk of diabetes-induced osteoporosis fracture.
Acknowledgement
This study was supported by Deputy of Research and Technology, Ilam University of Medical Sciences (No. 941030/166).
References
- Jackuliak P, Payer J. Osteoporosis, fractures and diabetes. Int J Endocrinol 2014; 820615: 1-10.
- De Waard E, van Geel T, Savelberg H, Koster A, Geusens P, van den Bergh G. Increased fracture risk in patients with type 2 diabetes mellitus: An overview of the underlying mechanisms and the usefulness of imaging modalities and fracture risk assessment tools. Maturitas 2014; 79: 265-274.
- Schwartz AV. Diabetes mellitus: does it affect bone? Calcif Tissue Int 2003; 73: 515-519.
- Milczarczyk A, Franek E. Osteoporosis and bone fractures in patients with diabetes mellitus. Diabet Dosw i Klin 2008; 8: 63-67.
- Hamann C, Kirschner S, Gunther KP, Hofbauer LC. Bone, sweet bone-osteoporotic fractures in diabetes mellitus. Nat Rev Endocrinol 2012; 8: 297-305.
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes-a meta-analysis. Osteoporos Int 2007; 18: 427-444.
- Schwartz AV, Vittinghoff E, Bauer DC, Hillier TA, Sterormeyer ES, Ensrud KE, Sterormeyer ES, Ensrud KE, Donaldson MG, Cauley AJ, Harris TB, Koster A, Womack CR, Palermo L, Black DM. Association of BMD and FRAX score with risk of fracture in older adults with type 2 diabetes. JAMA 2011; 305: 2184-2192.
- Ma L, Oei L, Jiang L, Estrada K, Chen H, Wang Z, Wang Z, Yu Q, Zillikens MC, Gao X, Rivadeneira F. Association between bone mineral density and type 2 diabetes mellitus: a meta-analysis of observational studies. Eur J Epidemiol 2012; 27: 319-332.
- Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 2007; 166: 495-505.
- Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabet Care 2001; 24: 1192-1197.
- Ivers RQ, Cumming RG, Mitchell P, Peduto AJ. Diabetes and risk of fracture: The Blue Mountains eye study. Diabet Care 2001; 24: 1198-1203.
- Dede A, Tournis S, Dontas I, Trovas G. Type 2 diabetes mellitus and fracture risk. Metabolism 2014; 63: 1480-1490.
- Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res 2007; 22: 1317-1328.
- Korpelainen R, Korpelainen J, Heikkinen J, Vaananen K, Keinanen-Kiukaanniemi S. Lifestyle factors are associated with osteoporosis in lean women but not in normal and overweight women: a population-based cohort study of 1,222 women. Osteoporos Int 2003; 14: 34-43.
- Kelsey JL, Browner WS, Seeley DG, Nevitt MC, Cummings SR. Risk factors for fractures of the distal forearm and proximal humerus: The study of osteoporotic fractures research group. Am J Epidemiol 1992; 135: 477-489.
- Melton LJ, Achenbach SJ, O'fallon WM, Khosla S. Secondary osteoporosis and the risk of distal forearm fractures in men and women. Bone 2002; 31: 119-125.
- Vogt MT, Cauley JA, Tomaino MM, Stone K, Williams JR, Herndon JH. Distal radius fractures in older women: a 10-year follow-up study of descriptive characteristics and risk factors: The study of osteoporotic fractures. J Am Geriatr Soc 2002; 50: 97-103.
- Van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A. Bone density in non-insulin-dependent diabetes mellitus. Ann Intern Med 1995; 122: 409-414.
- Heath H, Melton LJ, Chu CP. Diabetes mellitus and risk of skeletal fracture. N Engl J Med 1980; 303: 567-570.
- Tuominen JT, Impivaara O, Puukka P, Ronnemaa T. Bone mineral density in patients with type 1 and type 2 diabetes. Diabet Care 1999; 22: 1196-1200.
- Meyer HE, Tverdal A, Falch JA. Risk factors for hip fracture in middle-aged Norwegian women and men. Am J Epidemiol 1993; 137: 1203-1211.
- Forsen L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trøndelag Health Survey. Diabetologia 1999; 42: 920-925.
- Ottenbacher KJ, Ostir GV, Peek MK, Goodwin JS, Markides KS. Diabetes mellitus as a risk factor for hip fracture in Mexican-American older adults. J Gerontol A Biol Sci Med Sci 2002; 57: 648-653.
- de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA. Bone mineral density and fracture risk in type-2 diabetes mellitus: The Rotterdam Study. Osteoporos Int 2005; 16: 1713-1720.
- Gerdhem P, Isaksson A, Akesson K, Obrant KJ. Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos Int 2005; 16: 1506-1512.
- Strotmeyer ES, Cauley JA, Schwartz AV, Nevitt MC, Resnick HE, Bauer DC. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: The health, aging, and body composition study. Arch Intern Med 2005; 165: 1612-1617.
- Bonds DE, Larson JC, Schwartz AV, Strotmeyer ES, Robbins DC, Rodriguez BL, Janson KC, Margolis KL. Risk of fracture in women with type 2 diabetes: The women’s health initiative observational study. J Clin Endocrinol Metab 2006; 91: 3404-3410.
- Holmberg AH, Johnell O, Nilsson PM, Nilsson J, Berglund G. Akesson K. Risk factors for fragility fracture in middle age. A prospective population based study of 33,000 men and women. Osteoporos Int 2006; 17: 1065-1077.
- Dobnig H, Piswanger-Solkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, Maier E, Maritschnegg P, Sieberer C, Fahrleitner-Pammer A. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk. J Clin Endocrinol Metab 2006; 91: 3355-3363.
- Ahmed LA, Joakimsen RM, Berntsen GK, Berntsen GK, Fonnebo V, Schirmer H. Diabetes mellitus and the risk of non-vertebral fractures: The Tromsø Study. Osteoporos Int 2006; 17: 495-500.
- Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes mellitus and risk of hip fracture: The Nurses’ Health Study. Diabet Care 2006; 29: 1573-1578.
- Leslie W, Lix L, Prior H, Derksen SH, Metge C, O'Neil J. Biphasic fracture risk in diabetes: A population-based study. Bone 2007; 40: 1595-1601.
- Lipscombe L, Jamal S, Booth G, Kawker G. The risk of hip fractures in older individuals with diabetes. Diabet Care 2007; 30: 835-841.
- Melton LJ, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: Update of a population-based study. J Bone Miner Res 2008; 23: 1334-1342.
- Chen HF, Ho CA, Li CY. Increased risks of hip fracture in diabetic patients of taiwan. Diabet Care 2008; 31: 75-80.
- Mancini T, Mazziotti GH, Doga M, Carpinteri R, Simetovic N, Vescovi PP, Giustina A. Vertebral fractures in males with type 2 diabetes treated with rosiglitazone. Bone 2009; 45: 784-788.
- Yamamoto M, Yamaguchi T, Yamauchi M, Kaji H, Sugimoto T. Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 2009; 24: 702-709.
- Schneider A, Williams E, Brancati F, Blecker S, Coresh J, Selvin E. Diabetes and risk of fracture-related hospitalization: The atherosclerosis risk in communities study. Diabet Care 2013; 36: 1153-1158.
- Chung D, Choi H, Chung YS, Lim S, Yang SO, Shin CH. The prevalence and risk factors of vertebral fractures in Korean patients with type 2 diabetes. J Bone Miner Metab 2013; 31: 161-168.
- Oei L, Zillikens M, Dehghan A, Buitenijk G, Castano BM, Estrada K, Stolk L, Oei EH, van Meurs JB, Janssen JA, Hafman A, Leeuwen JP, Wittemen JC, Pols HA, Uitterlinden AG, Klaver CC, Franco OH, Rivadeneira F. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: The Rotterdam study. Diabet Care 2013; 36: 1619-1628.
- Reyes C, Estrada P, Nogués X, Orozco P, Cooper C, Díez-Pérez A, Formiga F, Gonzalez-Macias J, Prieto-Alhambra D. The impact of common co-morbidities (as measured using the Charlson index) on hip fracture risk in elderly men: a population-based cohort study. Osteoporos Int 2014; 25: 1751-1758.
- Napoli N, Strotmeyer ES, Ensrud KE, Sellmeyer DE, Bauer DC, Hoffman AR, Dam TT, Barrett-Connor E, Palermo L, Orwoll ES, Cummings SR, Black DM, Schwartz AV. Fracture risk in diabetic elderly men: the MrOS study. Diabetologia 2014; 57: 2057-2065.
- Leslie WD, Morin SN, Lix LM, Majumdar SR. Does diabetes modify the effect of FRAX risk factors for predicting major osteoporotic and hip fracture? Osteoporos Int 2014; 25: 2817-2824.
- Hothersall E, Livingstone SH, Looker H, Ahmed S, Cleland S, Leese G, Lindsay RS, McKnight J, Pearson D, Philip S, Wild SH, Colhoun HM. Contemporary risk of hip fracture in type 1 and type 2 diabetes: A National Registry Study From Scotland. J Bone Miner Res 2014; 29: 1054-1060.
- Looker AC, Eberhardt MS, Saydah SH. Diabetes and fracture risk in older US. adults. Bone 2016; 82: 9-15.
- Keegan TH, Kelsey JL, Sidney S, Quesenberry CP. Foot problems as risk factors of fractures. Am J Epidemiol 2002; 155: 926-931.
- Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral anti-diabetic medication on relative fracture risk. Diabetologia 2005; 48: 1292-1299.
- Seeman E. Growth in bone mass and size are racial and gender differences in bone mineral density are more apparent than real? J Clin Endocrinol Metab 1998; 83: 1414-1419.
- Kanazawa I, Yamaguchi T, Suqimoto T. Serum insulin-like growth factor-I is negatively associated with serum adiponectin in type 2 diabetes mellitus. Growth Horm IGF Res 2011; 21: 268-271.
- Baillie SP, Davison CE, Johnson FJ, Francis RM. Pathogenesis of vertebral crush fractures in men. Age Ageing 1992; 21: 139-141.
- Tan RS, Pu SJ. Impact of obesity on hypogonadism in the andropause. Int J Androl 2002; 25: 195-201.
- Barrett-Connor E, Khaw KT, Yen SS. Endogenous sex hormone levels in older adult men with diabetes mellitus. Am J Epidemiol 1990; 132: 895-901.
- Seeman E, Melton LJ 3rd, O’Fallon WM, Riggs BL. Risk factors for spinal osteoporosis in men. Am J Med 1983; 75: 977-983.
- Scane AC, Francis RM, Sutcliffe AM, Francis MJ, Rawlings DJ, Chapple CL. Case-control study of the pathogenesis and sequelae of symptomatic vertebral fractures in men. Osteoporos Int 1999; 9: 91-97.