Research Article - Biomedical Research (2020) Volume 31, Issue 3
The aqueous extract of Christ’s thorn (Ziziphus spina-christi) seed modulates hyperlipidemia in hypercholesterolemic male rat.
Abdulbasit I Al-Sieni1, Haddad A El Rabey2,3* and Madeha N Al- Seeni1
1Biochemistry Department, Faculty of Science, King Abdulaziz University, Jeddah, Saudi Arabia
2Biochemistry Department, Faculty of Science, University of Tabuk, Tabuk, Saudi Arabia
3Genetic Engineering and Biotechnology Institute, Sadat City University, Sadat City, PO Box 79, Menoufia, Egypt
- Corresponding Author:
- Haddad A El Rabey
Department of Biochemistry
University of Tabuk
Tabuk
Saudi Arabia
Accepted date: June 13, 2020
Abstract
The hypolipidemic and antioxidant activity of the crude aqueous extract of Christ’s thorn ( Ziziphus spina-christi ) seed was tested in hypercholesterolemic male rat. Eighteen albino rats were divided into 3 groups (n=6), the 1st group was the negative control fed fat rich diet, the 2ndwas the positive control fed 2% cholesterol in the fat rich diet and the 3rd was fed 2% cholesterol and concurrently treated with 200 mg/kg b.w. Christ’s thorn seed aqueous extract. The induced hypercholesterolemia in G2 increased lipid profile, lipid peroxide, liver and kidney function parameters, and decreased antioxidants. The tissues of kidney, liver, heart and testes showed altered histopathological changes. The concurrent treatment with Christ’s thorn seed aqueous extract in G3 restored the altered biochemical and histological features to normal. Phenolic constituents of Christ’s thorn seed aqueous extract suppressed the oxidative stress and reduced the hypercholesterolemia, and restored the altered biochemical and histopathological features.
Keywords
Hypercholesterolemia, Christ’s thorn, Seed, Antioxidant, Aqueous extract, Rats.
Introduction
Globally, hyperlipidemia has become a major cause of most serious diseases that threaten human health and life such as diabetes, high blood pressure coronary heart disease, stroke, peripheral vascular disease [1-3]. Hyperlipidemia is also considered a strong risk factor for atherosclerotic changes in the vascular wall [4,5].
Blood lipids control reduces the morbidity and mortality resulted from cardiovascular complication [6]. Exercise and supplementing diet with natural antioxidant can modulate lipid profile [3,7].
Zizyphusspina-christi (Christ ’ s thorn) of the family Rhamnaceae is commonly used in folk medicine in treating obesity, liver complaints, fever, urinary troubles, diabetes, diarrhea, digestive disorders, skin infections, weakness and insomnia [8,9]. The purified peptides from Z. jujuba proteins prevent oxidative reactions and can be used in medicinal purposes, and food preservation [10]. The leave extract of Zizyphusspina-christi revealed antidiabetic activity [9,11-13] by controlling meal-derived glucose absorption and has antioxidantand anti-inflammatory properties [14-17]. In addition, extracts of fruits and seeds of Z. spina-christi L. also revealed an antimicrobial activity against Bacillus subtilis, E. coli and Streptococcus pyogenes [18].
This study was focused in testing the effect of treating hypercholesterolemic male rats with Christ’s thorn seeds for 8 weeks to test their effect on lipid profile and antioxidants activity.
Materials and Methods
The Christ’s thorn seeds were collected from Christ’s thorn fruits from Jeddah, Saudi Arabia. All chemicals used are of analytical grade. All commercial kits used in the analysis were of international reputation.
Fat rich diet
The fat rich diet consisted of the following constituents: 64.5% Corn starch, 10% corn oil, 16% casein, 4% Salt Mixture, 4% N.N cellulose, 1% Vitamin Mixture, 0.2% DL, methionine and 0.2% choline chloride.
Christ’s thorn seed crude aqueous extract preparation
The Christ’s thorn seeds were washed with distilled water, sun dried for 72 h, and then milled using mixer. A 500 g of the powder were soaked in 5 litre distilled water for 72 h under constant shaking with intervals of 30 min. The mixture was filtered using 250 mm filter papers, and then the filtrate was freeze-dried at (-52°C). A 200 g semisolid product was produced. To obtain the recommended concentration, the suitable weight of the semisolid product was dissolved in the suitable amount of distilled water.
Experimental animals and experiment design
Eighteen Sprague Dawley male rats of East Asian origin were obtained from King Fahad Center for Medical Research, King Abdulaziz University, KSA. The rats were allocated in 3 stainless cages (n=6) and kept two weeks under observation for acclimatization prior the start of the experiment. All experiments were conducted in King Fahad Center for Medical Research under a protocol licensed by the bioethical committee of King Abdulaziz University. The rats were randomly divided into three groups as follows: the 1st negative control group (G1): normal rats fed fat rich diet, the 2nd positive control group (G2): fed 2% cholesterol in the fat rich diet to induce hypercholesterolemia [3,19,20], the 3rd group (G3): fed 2% cholesterol in the fat rich diet and concurrently treated with a daily dose of 200 mg/kg b.w. Christ’s thorn seed aqueous extract. The experiment was conducted for 8 weeks.
Blood collection
By the end of the experiment, animals were euthanized by cervical dislocation and blood samples were collected for serum preparation. The abdomen was dissected and the organs were rapidly dissected out. A piece of liver was kept cold in ice for liver tissue homogenate preparation.
Liver tissue homogenate
The liver tissue homogenate was prepared according to the method described in Al-Seeni et al.,[21].
The biochemical analyses
The biochemical analyses were carried out using the specified commercial kits from Human (Germany) according to the instructions of the suppliers.
The following tests were measured in serum: total cholesterol (TC), serum triglyceride (TG), serum high density lipoprotein (HDL). The serum low density lipoprotein (LDL) was calculated as follows: LDL (mg/dl)=total cholesterol–HDl– (triglycerides/5), whereas the very low density lipoprotein (VLDL)=triglycerides/5.
Liver enzymes; gamma-glutamyltransferase (γ-GT), alanine aminotransferase (ALT), and alkaline phosphatase (ALP), kidney function parameters; uric acid, creatinine, urea, and electrolytes: sodium, potassium, phosphorus and calcium ions were estimated in serum using Human (Germany) according to the instructions of the suppliers.
Antioxidants and lipid peroxidation
The antioxidants enzymes; glutathione reductase (GR), catalase, and superoxide dismutase (SOD) were estimated in liver tissue homogenate using Biodiagnostic (Egypt) according to the instructions of the suppliers.
Lipid peroxidation was estimated by measuring the malondialdehyde (MDA)-the final product of the unsaturated fatty acids in the homogenate of the liver tissue using Biodiagnostic kit (Egypt) according to the instructions of the supplier.
Histopathology
A piece of the liver, a kidney, a testis and the heart were washed in sterile saline and fixed in 10% formalin, dehydrated in an ascending series of ethanol (50%-99%), cleared in xylene, and then embedded in paraffin. 5 microtomes sections were stained in hematoxylin and eosin (H&E) dye, air dried, and then mounted in Canada balsam. The prepared sections were examined and photographed under a light microscope with a digital camera.
Statistical analysis
The SPSS (Statistical Program for Sociology Scientists) software Version 23.0 was used in analyzing data for computing the mean, the standard errors (SE) and test of significance (T-test).
Results and Discussion
Lipid profile
Hypercholesterolemia in G2 significantly increased serum total cholesterol, triglycerides, low density lipoprotein and very low density lipoprotein and decreased the useful high density lipoprotein as shown in Table 1. The concurrent treatment with Christ’s thorn seed aqueous extract significantly ameliorated all altered lipid parameters and nearly restored them to the normal levels as in G1.
| Parameters | Statistics | G1 Negative control |
G2 Positive control |
G3 Christ’s thorn seeds |
|---|---|---|---|---|
| TC | Mean ± SE | 100.27 ± 4.99 | 128.35 ± 1.22 | 71.86 ± 7.71 |
| (mg %) | T.test | -5.36*** | 7.50*** | |
| TG | Mean ± SE | 81.41 ± 1.60 | 117.07 ± 6.56 | 34.21 ± 3.40 |
| mg/dl | T.test | -5.09*** | 11.36*** | |
| HDL | Mean ± SE | 42.70 ± 3.13 | 40.16 ± 1.49 | 41.01 ± 0.88 |
| mg/dl | T.test | 0.66* | -0.52* | |
| LDL | Mean ± SE | 43.66 ± 2.47 | 46.35 ± 1.00 | 29.95 ± 7.98 |
| mg/dl | T.test | -0.79** | 2.04*** | |
| VLDL | Mean ± SE | 16.28 ± 0.32 | 23.32 ± 1.30 | 16.84 ± 0.68 |
| mg/dl | T.test | -5.06*** | 1.37*** |
Significant differences with controls calculated by paired sample t-test; *p<0.05, **p<0.01, ***p<0.001.
Table 1: Effect of Christ’s thorn seed aqueous extract on serum lipids in hypercholesterolemic male rats.
Antioxidant enzymes and lipid peroxidation
Table 2 shows that the antioxidant enzymes catalase, superoxide dismutase and glutathione reductase were increased in liver tissue homogenate as a result of induced hypercholesterolemia compared to the negative control, whereas lipid peroxidation was significantly increased. The concurrent administration of Christ’s thorn seed aqueous extract in G3 significantly increased the antioxidant enzymes under study and decreased the lipid peroxidation to the normal levels in G1.
| Parameters | Statistics | G1 Negative control |
G2 Positive control |
G3 Christ’s thorn seeds |
|---|---|---|---|---|
| Catalase U/I |
Mean ± SE | 258.50 ± 15.65 | 113.00 ± 2.768 | 242.50 ± 3.20 |
| T.test | 10.855*** | -9.66*** | ||
| Superoxide dismutase U/ml |
Mean ± SE | 262.63 ± 16.58 | 245.50 ± 29.01 | 295.22 ± 14.57 |
| T.test | 0.53*** | -1.53*** | ||
| Glutathione reductase U/ml |
Mean ± SE | 16.98 ± 0.69 | 14.78 ± 0.39 | 6.95 ± 0.35 |
| T.test | 2.78 ** | 14.25 *** | ||
| Lipid peroxidase nmol/g tissue |
Mean ± SE | 626.77 ± 28.05 | 792.33 ± 31.28 | 228.50 ± 21.11 |
| T.test | -4.23*** | 13.38*** |
Significant differences with controls calculated by paired sample t-test; *p<0.05, **p<0.01, ***p<0.001.
Table 2: Effect of treating hypercholesterolemic rats with Christ’s thorn seed aqueous extract for 8 weeks on antioxidants and lipid peroxidation in liver tissue homogenate.
Liver function
Induction of hypercholesterolemia in G2 increased all liver function enzymes; ALT, AST and γ-GT in serum compared to the negative control as shown in Table 3. While the concurrent administration of the aqueous extract of Christ’s thorn seed in G3 significantly restored the liver enzymes to their normal levels.
| Parameters | Statistics | G1 Negative control |
G2 Positive control |
G3 Christ’s thorn seeds |
|---|---|---|---|---|
| ALT U/l) ) γ-GT U/l) |
Mean ± SE | 23.61 ± 1.59 | 41.50 ± 5.24 | 24.00 ± 0.68 |
| T.test | -3.49** | 3.48** | ||
| Mean ± SE | 3.75 ± 0.18 | 4.28 ± 0.47 | 3.46 ± 0.20 | |
| T.test | -0.94 | 1.78* | ||
| ALP U/l) |
Mean ± SE | 61.78 ± 13.36 | 91.66 ± 3.98 | 71.46 ± 4.43 |
| T.test | -2.16* | 3.24** |
Significant differences with controls calculated by paired sample t-test; NS: Nonsignificant*p<0.05 **p<0.01.
Table 3: Effect of Christ’s thorn seed aqueous extract on liver enzymes in hypercholesterolemic male rats.
Kidney function and serum electrolytes
Kidney function parameters were nonsignificantly increased as a result of induced hypercholesterolemia in G2 compared to the negative control as shown in Table 4, whereas the concurrent treating of the hyepercholesterolemic male rats in G3 with Christ’s thorn seed aqueous extract restored the studied kidney function parameters to the normal.
| Parameters | Statistics | G1 Negative control |
G2 Positive control |
G3 Christ’s thorn seeds |
|
|---|---|---|---|---|---|
| Uric acid mg/dl | Mean ± SE | 2.63 ± 0.11 | 2.70 ± 0.46 | 1.36 ± 0.07 | |
| T.test | -0.15 NS | 3.06** | |||
| Creatinine mg/dl | Mean ± SE | 0.74 ± 0.02 | 0.76 ± 0.02 | 0.25 ± 0.01 | |
| T.test | -0.60 NS | 19.27*** | |||
| Urea mg/dl |
Mean ± SE | 31.86 ± 5.174 | 40.65 ± 2.86 | 27.23 ± 6.44 | |
| T.test | -1.625 NS | 2.63** | |||
| Sodium mmol/l | Mean ± SE | 136.53 ± 7.93 | 156.78 ± 6.10 | 185.17 ± 12.51 | |
| T.test | -1.75 NS | -1.75 NS | |||
| Potassium mmol/l |
Mean ± SE | 4.78 ± 0.46 | 5.01 ± 0.27 | 5.80 ± 0.42 | |
| T.test | -0.37 NS | -1.37 NS | |||
| Phosphorus mg/dl |
Mean ± SE | 4.60 ± 0.48 | 4.98 ± 0.67 | 6.12 ± 0.21 | |
| T.test | -0.47 NS | -1.61 NS | |||
| Calcium mg/dl | Mean ± SE | 9.83 ± 0.21 | 8.61 ± 0.37 | 15.45 ± 0.31 | |
| T.test | 2.37* | -10.92*** | |||
Significant differences with controls calculated by paired sample. t-test; NS: non significant, *p<0.05, **p<0.01, ***p<0.001.
Table 4: Effect of Christ ’ s thorn seed aqueous extract on kidney function in hypercholesterolemic male rats.
Table 4 shows also that in G2, the induced hypercholesterolemia nonsignificantly increased serum sodium, potassium and phosphorus ions, whereas significantly decreased calcium ions compared to G1. Treating hypercholesterolemia in G3 with Christ’s thorn seed aqueous extract, non-significantly affect serum sodium, potassium and phosphorus ions, whereas significantly increased calcium ions.
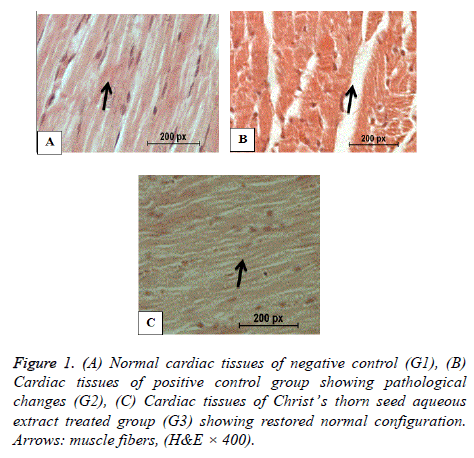
Histopathology of heart
Figure 1A shows the normal structure of the cardiac muscles of the negative control. Figure 1B shows damaged cardiac muscles and necrotic muscle fibres with increased hyalinization of the hypercholesterolemic rats of G2. Figure 1C shows nearly normal cardiac muscles of the hypercholesterolemic rats of G3 treated with Christ’s thorn seed aqueous extract.
Total body weight
Table 5 shows that induced hypercholesterolemia in G2 significantly increased total body weight, whereas treating the hypercholesterolemic male rats in G2 significantly reduced the weight approaching nearly the normal values of G1.
| Parameters | Statistics | G1 | G2 | G3 |
|---|---|---|---|---|
| Negative control | Positive control | Christ’s thorn seeds | ||
| Initial weight | Mean ± SE | 151.50 ± 2.58 | 151.67 ± 3.33 | 151.80 ± 1.59 |
| T.test | -3.95 NS | 3.37 NS | ||
| 1st week | Mean ± SE | 161.67 ± 3.07 | 175.00 ± 5.62 | 162.66 ± 2.07 |
| T.test | -2.00 *** | 2.69*** | ||
| 2nd week | Mean ± SE | 181.67 ± 1.66 | 206.67 ± 3.33 | 183.61 ± 1.12 |
| T.test | -7.31*** | 5.83*** | ||
| 3rd week | Mean ± SE | 201.67 ± 1.66 | 205.00 ± 2.23 | 204.67 ± 2.16 |
| T.test | -4.00** | 6.32*** | ||
| 4th week | Mean ± SE | 211.67 ± 1.66 | 215.00 ± 2.23 | 212.65 ± 1.61 |
| T.test | -1.58 ** | 1.00 NS | ||
| 5th week | Mean ± SE | 218.33 ± 1.66 | 231.67 ± 4.01 | 220.38 ± 1.16 |
| T.test | -2.69** | 2.69** | ||
| 6th week | Mean ± SE | 235.00 ± 4.28 | 246.67 ± 3.33 | 239.08 ± 4.28 |
| T.test | -3.79** | 3.79** | ||
| 7th week | Mean ± SE | 243.33 ± 8.81 | 265.00 ± 7.18 | 248.36 ± 8.81 |
| T.test | -1.45*** | 1.45*** | ||
| 8th week | Mean ± SE | 261.67 ± 14.24 | 280.00 ± 16.93 | 251.77 ± 16.00 |
| T.test | -2.10*** | 1.47*** |
Significant differences with controls calculated by paired sample t-test; NS: Nonsignificant, **p<0.01, ***p<0.001.
Table 5: Effect of Christ’s thorn seed aqueous extract administration on total body weight of male rats under study.
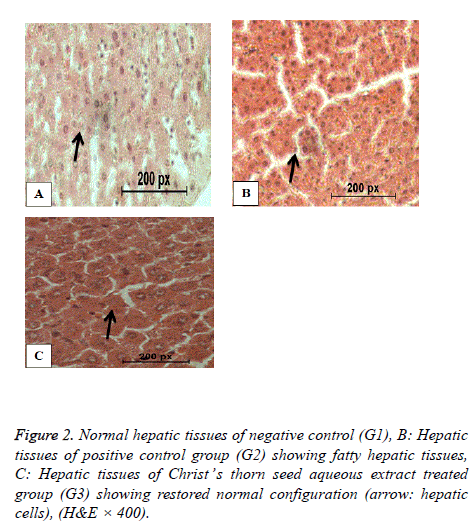
Histopathology of liver
Figure 2 shows the normal hepatic tissues of the negative control group G1 with normal cells and blood sinusoids. Figure 2 shows fatty liver of the positive control group (G2) with disrupted hepatic cell strands and vacuolated cytoplasm and necrosis. Figure 2 shows nearly normal hepatic tissues of hypercholesterolemic rats of G3 treated with Christ’s thorn seed aqueous extract with normal cell strands and nuclei.
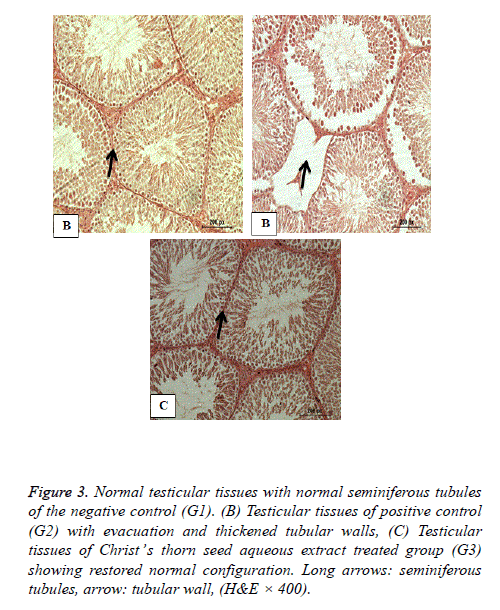
Histopathology of testes
Figure 3 shows the normal testicular tissues of the negative control group (G1) with regular seminiferous tubules. Figure 3 shows severely evacuated seminiferous tubules with thickened tubular walls and decreased germinal cells of the hypercholesterolemic positive control G. Figure 3 shows nearly normal testicular structure of hypercholesterolemic rats of the third group fed 2% cholesterol and co treated with Christ’s thorn seed aqueous extract for 8 weeks with restored normal structure.
Figure 3: Normal testicular tissues with normal seminiferous tubules of the negative control (G1). (B) Testicular tissues of positive control (G2) with evacuation and thickened tubular walls, (C) Testicular tissues of Christ’s thorn seed aqueous extract treated group (G3) showing restored normal configuration. Long arrows: seminiferous tubules, arrow: tubular wall, (H&E × 400).
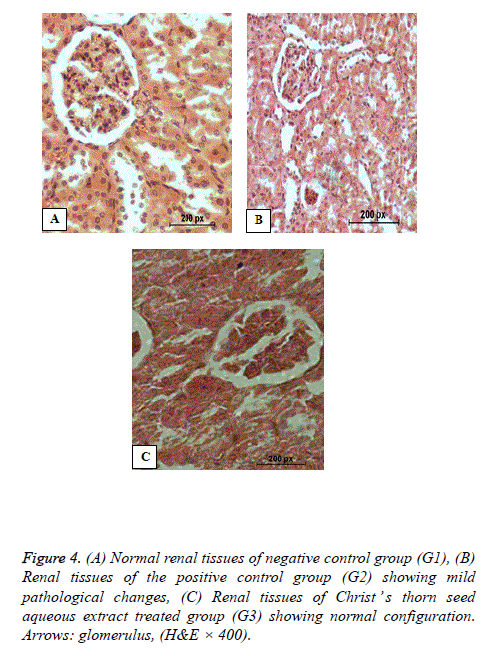
Histopathology of kidney
Figure 4 shows the normal renal tissues of the negative control (G1) with normal renal pattern, uriniferous tubules with regulated nuclear arrangement and normal glomerulus. Figure 4 shows mildly altered renal tissues of the hypercholesterolemic rats of G2 with mildly dilated and shrinkage glomeruli. Figure 4 shows nearly normal renal tissues of G3 hypercholesterolemic rats that were concurrently treated with Christ’s thorn seed aqueous extract for 8 weeks with nearly restored normal renal tissues.
In the present study, the hypolipidemic and antioxidant activity of Christ’s thorn seed aqueous extract was investigated in hypercholesterolemic male rats. Induced hypercholesterolemia elevated lipid profile parameters [3,20,21]. While, treating hypercholesterolemic rats with Christ’s thornseed aqueous extract ameliorated the lipid parameters by decreasing the total cholesterol, triglycerides LDL and VLDL, and increased the HDL compared to the positive control hypercholesterolemic group. The leaf powder and extract of Christ’s thorn revealed a similar hypolipidemic effect [8]. This bioactive antioxidant and hypolipidemic activity of Christ’s thorn crude aqueous extract may be due to its constituents; gallocatechin, epigallocatechin, 6’’' sinapoylspinosin, spinosin, 6’’’ feruloylspinosin [22].
Similarly, the induced hypercholesterolemia increased lipid peroxidation as revealed by the increase in the malondialdehyde (MDA) content in the liver tissue homogenate, whereas the antioxidant enzymes; superoxide dismutase, catalase and Glutathione reductase were decreased. This result is in congruent with previous studies [3,21].
The concurrent administration of Christ’s thorn crude seed aqueous extract also suppressed the oxidative stress by decreasing lipid peroxidation and increasing the antioxidant enzymes activity due to its active bio-constituents [22].
The activity of ALPAL and γ-GT representing liver function in this study was also increased in the hypercholesterolemic male rats of the positive group [3].These liver function enzymes restored their normal activities by administrating Christ’s thorn crude seed aqueous extract which is consistent with Dkhil et al., [16] who stated that Christ’s thorn methanolic leaf extract inhibited liver and heart injury. The kidney function parameters were nonsignificantly affected by the induced hyperlipidemiain the positive control [4,7,23]. On the line of the other studied parameters, administration of Christ’s thorn seed aqueous extract ameliorated the levels of creatinine, uric acid and urea in the serum.
Similarly, serum electrolytes were also nonsignificantly affected by the induced hypercholesterolemiain in the positive control due to the altered membrane permeability or glomerular filtration rate [24]. Administration of Christ’s thorn seed aqueous crude extractameliorated thelevels of all electrolytes and restored the normal levels. This therapeutic effect of the aqueous extract of Christ’s thorn seeds may be due to its antioxidant and anti-inflammatory activity [13,16,22].
The histology of the studied organs reflected the altered biochemical investigations is in congruent with previous studies; the liver was severely affected [2,21]. Also the heart [3,21] and testes [8,25] were severely injured whereas the kidney was mildly affected [7] by the induced hypercholesterolemia in the positive control. However, Bentley et al., [4] stated that hypercholesterolemia causes renal morphological damage due to the increased microvascular density in the renal cortex that may lead to real disease progression.
This result is consistent with similar studies confirmed a correlation between hypercholesterolemia and alteration in organ’s histology [21,24-26]. The concurrent administration of Christ’s thorn crude seed aqueous extract alleviated these histological changes of the studied organs and restored their normal histology. This alleviating activity of Christ’s thorn seed aqueous extract is due to its antioxidant and freed radical scavenging activity of the phenolic constituents of the seed aqueous extract which helped in suppressing oxidative stress that lead to cell damage and reduced the effect of hypercholesterolemia [13].
In addition, total body weight was significantly increased by hyperlipidemia [21] and restored its normal weight as in the negative control with the concurrent treatment with Christ’s thorn seed aqueous extract.
Conclusion
In conclusion, induction of hypercholesterolemia severely altered lipid profile, liver function, oxidative stress, and histology of liver, heart, testis and kidney. While treating the hypercholesterolemic male rats with Christ ’ s thorn seed aqueous extract restored all biochemical blood tests and the histology of the studied organs early to the normal. This may be attributed to the antioxidant and inhibition of oxidative stress by the phenolic constituents of the Christ’s thorn seed.
Conflict of Interest
The authors declare that they have no conflict of interests.
References
- Nelson RH. Hyperlipidemia as a risk factor for cardiovascular disease. Primary Care: Clini Office Pract 2013; 40: 195-211.
- Abulnaja KO, El Rabey HA. The Efficiency of Barley (Hordeumvulgare) Bran in Ameliorating Blood and Treating Fatty Heart and Liver of Male Rats. Evidence-Based Complement Alternat Med 2015; 1: 13.
- El Rabey HA, Al-Seeni MN, Al-Ghamdi HB. Comparison between the hypolipidemic activity of parsley and carob in hypercholesterolemic male rats. BioMed Res Int 2017; 2: 17.
- Bentley MD, Rodriguez-Porcel M, Lerman A, Sarafov MH, Romero JC, Pelaez LI, Grande JP, Ritman EL, Lerman LO. Enhanced renal cortical vascularization in experimental hypercholesterolemia. Kidney Int 2002; 61: 1056-1063.
- Makni M, Fetoui H, Gargouri NK, Garoui EM, Jaber H, Makni J, Boudawara T, Zeghal N. Hypolipidemic and hepatoprotective effects of flax and pumpkin seed mixture rich in ω-3 and ω-6 fatty acids in hypercholesterolemic rats. Food ChemToxicol 2008; 46: 3714-3720.
- Amundsen ÅL, Ose L, Nenseter MS, Ntanios FY. Plant sterol ester–enriched spread lowers plasma total and LDL cholesterol in children with familial hypercholesterolemia. The Am J ClinNutri 2002; 76: 338-344.
- El Rabey HA, Al-Seeni MN, Amer HM. Efficiency of barley bran and oat bran in ameliorating blood lipid profile and the adverse histological changes in hypercholesterolemic male rats. BioMed Res Int 2013; 1: 2013.
- El Rabe HA, Attia ES, Al-Seeni MN, Al-Sieni AI, Ibrahim IH, Meerasahib MF, Shaikh-Omer AM, Abuelgassim AO. The hypolipidemic and antioxidant activity of Christ’s thorn (Ziziphus spina-christi) leaves powder in hypercholesterolemic male rats. Life Sci J 2014; 11: 1010-1021.
- Al-Awar MS. Anti-diabetic Activities of Zizyphusspina-christi Seeds Embryos Extract on General Characteristics of Diabetes, Carbohydrate Metabolism Enzymes and Lipids Profile in Rats. Jordan J Pharm Sci 2019; 12: 2.
- Memarpoor-Yazdi M, Mahaki H, Zare-Zardini H. Antioxidant activity of protein hydrolysates and purified peptides from Zizyphusjujuba fruits. J Fun Food 2013; 5: 62-70.
- Michel CG, Nesseem DI, Ismail MF. Anti-diabetic activity and stability study of the formulated leaf extract of Zizyphusspina-christi (L.) Willd with the influence of seasonal variation. J Ethnopharmacol 2011; 133: 53-62.
- Asgarpanah J, Haghighat E. Phytochemistry and pharmacologic properties of Ziziphusspinachristi (L.) Willd. African J Pharm Pharmacol 2012; 6: 2332-2339.
- Almeer RS, El-Khadragy MF, Abdelhabib S, Moneim AE. Ziziphus spina-christi leaf extract ameliorates schistosomiasis liver granuloma, fibrosis, and oxidative stress through downregulation of fibrinogenicsignaling in mice. PloS one 2018; 13: 10.
- Al-Reza SM, Yoon JI, Kim HJ, Kim JS, Kang SC. Anti-inflammatory activity of seed essential oil from Zizyphusjujuba. Food ChemToxicol 2010; 48: 639-643.
- Abdallah EM, Elsharkawy ER, Ed-Dra A. Biological activities of methanolic leaf extract of Ziziphusmauritiana. Pharm CommunBiosci Biotech Res Comm 2016; 9: 605-614.
- Dkhil MA, Al-Quraishy S, Moneim AE. Ziziphus spina-christi leaf extract pretreatment inhibits liver and spleen injury in a mouse model of sepsis via anti-oxidant and anti-inflammatory effects. Inflammopharmacol 2018; 26: 779-791.
- Dkhil MA, Kassab RB, Al-Quraishy S, Abdel-Daim MM, Zrieq R, Moneim AE. Ziziphus spina-christi (L.) leaf extract alleviates myocardial and renal dysfunction associated with sepsis in mice. Biomed Pharmacother 2018; 102: 64-75.
- Nazif NM. Phytoconstituents of Zizyphusspina-christi L. fruits and their antimicrobial activity. Food Chem 2002; 76: 77-81.
- Ónody A, Csonka C, Giricz Z, Ferdinandy P. Hyperlipidemia induced by a cholesterol-rich diet leads to enhanced peroxynitrite formation in rat hearts. Cardiovascular Res 2003; 58: 663-70.
- Rathod DM, Dodiya HG, Goswami SS. Experimentally-induced Hyperlipidemia. Int J Pharmacol 2011; 7: 690-696.
- Liu ZM, Ho SC, Chen YM, Xie YJ, Huang ZG, Ling WH. Research protocol: effect of natural S-equol on blood pressure and vascular function-a six-month randomized controlled trial among equal non-producers of postmenopausal women with prehypertension or untreated stage 1 hypertension. BMC Complementary and Alternative Med 2016; 16: 89.
- Kadioglu O, Jacob S, Bohnert S, Naß J, Saeed ME, Khalid H, Merfort I, Thines E, Pommerening T, Efferth T. Evaluating ancient Egyptian prescriptions today: anti-inflammatory activity of Ziziphus spina-christi. Phytomed 2016; 23: 293-306.
- Ghule AE, Jadhav SS, Bodhankar SL. Trigonelline ameliorates diabetic hypertensive nephropathy by suppression of oxidative stress in kidney and reduction in renal cell apoptosis and fibrosis in streptozotocin induced neonatal diabetic (nSTZ) rats. IntImmunopharmacol 2012; 14: 740-748.
- El-Missiry MA, Othman AI, Amer MA, Abd El-Aziz MA. Attenuation of the acute adriamycin-induced cardiac and hepatic oxidative toxicity by N-(2-mercaptopropionyl) glycine in rats. Free Radical Res 2001; 35: 575-581.
- Ouvrier A, Alves G, Damon-Soubeyrand C, Marceau G, Cadet R, Janny L, Brugnon F, Kocer A, Pommier A, Lobaccaro JM, Drevet JR. Dietary cholesterol-induced post-testicular infertility. PloS one 2011; 6: 11.
- Dimitrova-Shumkovska J, Veenman L, Ristoski T, Leschiner S, Gavish M. Chronic high fat, high cholesterol supplementation decreases 18 kDaTranslocator Protein binding capacity in association with increased oxidative stress in rat liver and aorta. Food Chem Toxicol 2010; 48: 910-921.