Research Article - Biomedical Research (2017) Volume 28, Issue 11
Tetrandrine reverses the drug resistance of colon cancer to 5-fluorouracil
Kai-lei Wang1,2, Li-na Ma3, Guang-bo Bu2, Gang Ma2, Guang-dong Zhang2, Le-ping Li1, Chang-qing Jing1 and Cheng-kun Qin1*
1Department of General Surgery, Provincial Hospital of Shandong University, 324#, Jing 5 Road, Jinan City 250012, PR China
2Department of General Surgery, Teng Zhou Center People Hospital, 181#, Xingtan Road, Tengzhou City 277500, PR China
3Department of Obstetrics and Gynecology, Teng Zhou Center People Hospital, 181#, Xingtan Road, Tengzhou City 277500, PR China
- *Corresponding Author:
- Cheng-kun Qin
Department of General Surgery
Provincial Hospital of Shandong University, PR China
Accepted date: March 25, 2017
Abstract
Aim: Treatment for colon cancer is based mainly on the different stage of the cancer and chemotherapy is used as additional therapeutic approaches. 5-Fluorouracil (5-Fu) treatment is a conventional chemotherapeutical drug for colorectal cancer. However, a large amount of patients cannot be benefit from this therapy due to the resistance against 5-Fluorouracil displayed by their tumor. Tetrandrine (TET), a bisbenzylisoquinoline alkaloid, exerts antitumor effects on certain kinds of cancers. Here, we elucidate the potential application of tetrandrine to eradicate the resistance of colon cancer against 5- Fluorouracil.
Methods: Colon cancer LOVO cell line and its 5-Fu-resistanct variant, LOVO/5-Fu, were employed in this study. Those two types of cells were treated with TET for 48 hours. Afterwards, cell viability was measured by MTT colorimetric assay. Cell cycle and apoptosis were determined by flow cytometry assay. P-glycoprotein (P-gp) is believed playing a major role in the anti-drug effect of tumor cells. Thus the expression of P-gd both in mRNA and protein level were analyzed with quantitative PCR and Western blot respectively.
Results: After LOVO/5-Fu cells were treated with TET for 48h, the IC50 of 5-Fu was significantly decreased to 4.15 ± 0.31 μg/ml (P<0.05); and the apoptotic rate were increased to 3.82% ± 0.12% (P<0.05). Coincidently, the expression of P-gp mRNA was significantly decreased to 570 ± 85 (P<0.05) and protein level of P-gp was also reduced respectively.
Conclusions: TET increased the sensitivity and reversed the drug-resistance of colon carcinoma cells to 5-Fu, which is relied on a P-gp-dependent manner.
Keywords
Colorectal neoplasm, Tetrandrine, Multidrug resistance, P-glycoprotein
Introduction
Colon cancer is one of the most common cancers worldwide, resulting in 639,000 deaths every year. Recently, there was significant soaring morbidity and mortality caused by colon cancer in China. Therefore, the treatment for colon cancer has become a task of priority in modern medicine. At present, several therapeutic approaches are available for colon cancer, including surgery, radiation therapy and chemotherapy. In spite of a well-designed treatment, namely surgery combined with adjuvant chemotherapy, recurrence rate is as high as 33% [1]. The development of multiple drug resistance (MDR) is the major issue that affecting the efficacy of cancer chemotherapy. Therefore, exploring for novel and effective strategies against drug resistance is imperative necessity. Several non-cytotoxic drugs, including verapamil and phenothiazines, have been shown to attenuate MDR partly depending on competitively inhibiting P-gp-mediated drug efflux [2]. However the effective concentration of verapamil and phenothiazines against MDR are harmful for patients with the side effects including heart block and central nerve system (CNS) depression.
Tetrandrine (TET), a bisbenzylisoquinoline alkaloid, is extracted from the root of the creeper Stephania tetrandra. It is reported that TET has been applied as an antifibrotic drug to treat silicosis in China in the 1960s. It exhibited minor toxic to humans, even at a dosage of 180 mg at three-times per day [3]. TET has already been employed in traditional Chinese medicine for several decades during the treatment of arthritis, silicosis and hypertension [4]. Recently, tetrandrine has also been shown to function as an antitumor molecule. TET could inhibit the proliferation and induce the apoptosis of various cancer cells, such as breast cancer, hepatoma, lung cancer, glioma and leukemia cells [5-9]. Interestingly, in addition to repressing proliferation and promoting apoptosis rate of a broad range of cancer cells, TET also exhibited impact on drug-resistant cancer cell lines. The drug resistant properties of TET is making it as a promising adjunct drug to chemotherapy in the context of MDR [10,11]. Specifically, 5-fluorouracil (5- Fu) is widely used in chemotherapy of colon cancers. In the present study, we analyzed the impact of a combination of TET and 5-Fu on human colon carcinoma multidrug-resistant LOVO/5-Fu cells and also examined the possibility of TET as an adjuvant to optimize the therapeutic effect of 5-Fu on drugresistance of colon cancers.
Materials and Methods
Reagents
The compound, TET (C38H42O6N2), was purchased from the National Institute for the Control of Pharmaceutical and Biological Produces (Beijing, China). 5-FU was obtained from the Shanghai Reagent Factory (Shanghai, China). Cell RNA was lysis with Trizol reagent (Invitrogen, Carlsbad, CA, USA). cDNA was reverse with the TaKaRa kit (Dalian, China) following the instruction guide. Apoptosis cells were determined by Annexin-V analysis (Pharmingen, San Diego, CA, USA). Antibodies used in the immunoblot analysis, including those specific for phosphorylated glycoprotein and β- actin were from Abcam (Cambridge, UK).
Cell lines
LOVO cells (the human colon adenocarcinoma cell line) and LOVO/5-FU (fluorouracil-resistant cell subline of human colon adenocarcinoma) were cultured in the RPMI 1640 medium (Sigma, USA), which were supplemented with 10% fetal bovine serum, streptomycin (100 U/ml) and penicillin (100 U/ml). Those cells were maintained at 37°C in a humidified atmosphere of 5% CO2 cell culture incubator. The LOVO/5-FU were purified by stepwise selection in increasing concentrations of 5-FU, starting with the concentration of 0.01 μg/ml. When cells became confluent in medium supplied with 5-FU, the drug concentration was increased from 0.03, 0.05, 0.1, 0.3, 0.5, 1, 1.5 to 2.5 μg/ml. The maximal concentration used in the system was 2.5 μg/ml. The LOVO/5-FU cell subline was passaged in the fluorouracil-free medium and remained stably resistant to 5-FU around several months. The cells were periodically reselected in the presence of 2.5 μg/ml 5-FU in order to prevent the outgrowth of revertants. Using these conditions to maintain the stability of cell lines and no change in resistance was observed over 1 year.
MTT Assay
Cell viability was evaluated using the MTT assay. The cells were dispensed in 96-well flat bottom plates with the density of 5 × 104 cells per well. After incubation for 24 h, they were treated with different concentration of TET and/or fluorouracil and then were cultured for another 48 h. After stimulation, the cells were incubated with MTT (0.25 mg/ml) for 4 h at 37°C in the incubator and the absorbance at 570 nm was measured using uquant MQX 200 microplate reader (Bio-tek, USA). This inhibition rate was determined by triplicate assays. The half of inhibition concentrations (IC50) values were calculated with the cytotoxicity curves. The resistance index (RI) was calculated via dividing the IC 50 with the MDR cells.
Cell cycle analysis
Colon cells (1 × 106) were seeded in 6-well plates for around 24 h. After the culture medium was replaced with fresh medium and stimulated with TET for another 48 h. Then the cells were harvested by typsinization digestion and were washed with cold PBS among twice times. Those cells were fixed with 70% cold ethanol overnight at 4°C room. Cell cycle were determined by staining with a propidium iodide (PI) solution which consisting of 10 μg/ml PI, 0.1% Triton X-100, and 250 μg/ml RNase A. Those staining cells were incubated at room temperature for 1 h, after that, those cells were test with flow cytometer and were analysis with Cell Quest 3.1 software (Becton Dickinson, CA, USA). Each sample was analysed with three replicates. Cell cycle distributions were calculated with Multicycle software.
Flow cytometry
The apoptosis rate of cells was measured with annexin V-FITC apoptosis detection kit. Briefly, after detached by trypsin treatment, the cells were resuspended in PBS with the concentration of 105 cells/ml, and then labeled with Annexin V-FITC for about 10 min. Once after addition of 10 μl PI, the cells were analyzed by flow cytometry immediately. Detection of the double positive cells with flow cytometry.
Quantitative reverse transcription-PCR
Total RNA was extracted from cells using Trizol Reagent (Invitrogen, USA) and followed by the manufacturer’s protocol. The cDNA was carried out by a PrimeScriptTM RT Reagent kit (TaKaRa, China). Primers used in RT-PCR were as follows: MDR1 forward: 5'- CCCATCATTGCAATAGCAGG-3' and reverse: 5'- GTTCAAACTTCTGCTCCTGA-3'; β-actin forward: 5'- CTTCTACAATGAGCTGCGTG-3' and reverse: 5'- TCATGAGGTAGTCAGTCAGG-3'. The expression of RNA level was standardized with β-actin respectively. Each sample was tested with triplicate times. Then the amplified PCR products were electrophoresed on 1% agarose gels, and PCR fragments were visualized by ethidium bromide staining and quantified with Tanon GIS2000 gel image software.
Western blotting
The expression levels of p-gp and actin proteins were determined using western blotting. After the cells were stimulated with or without tetrandrine for 48 h, they were washed with PBS, and lysis with RIPA Lysis Buffer (Beyotime, China) for about 20 min. Next the supernatant was collected after centrifuging at 12,000 g for 15 min. The concentration of protein was detected by using a BCA Protein Assay Kit (Beyotime, China). Then protein extracts were fractionated on 12% polyacrylamide sodium dodecyl sulfate (SDS) gel and were transferred to nitrocellulose membrane. The membrane was blocked with 5% (w/v) fat-free milk for 1h, followed by incubation with the specific rabbit primary polyclonal antibody at 4°C overnight. After the membrane was treated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody for 1 h, the membranes were visual with ECL Western Blotting Detection Reagent.
Statistical analyses
Statistical analysis was performed using SPSS15.0 software. Data are presented as means ± S.E.M., and the significance was assessed by unpaired two-tailed t-test unless otherwise indicated. A value of p<0.05 was considered as significantly different from the control.
Results
The effect of TET on cell viability of 5-FU-resistant cancer cells
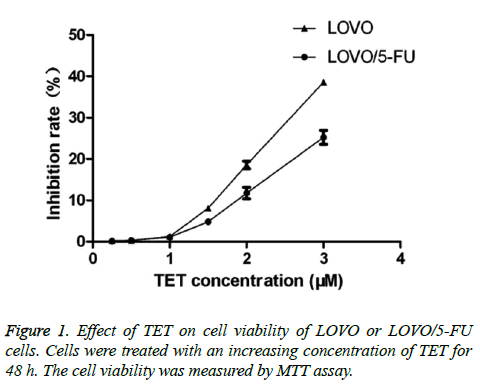
To explore the effect of TET on the certain cancer cells, we first examined the cytotoxicity of TET itself on both the 5-FU - resistant cell line (LOVO/5-FU) and its parental cell line (LOVO). During the experiment, both of those cancer cell lines were firstly pretreated with a titrated concentration of TET from 0.25 μM to 3 μM for around 48 h. TET was found to significantly inhibit the viability of both LOVO/5-FU and LOVO cell lines at a relatively high concentration. While the LOVO/5-FU and LOVO cells were significantly inhibited by tetrandrine at the concentration of 2 μM of TET. Cell viability was always higher than ninety percentage in both LOVO/5-FU and LOVO cells when the cell were exposed to 1 μM or even lower concentrations of TET. Importantly, at this concentration there is no significant difference between the cell viability of LOVO/5-FU and LOVO cells (Figure 1). Therefore, the concentrations of TET were employed as 1.0 μM during the following experiment.
The reversal effect of TET on 5-Fu-resistant cancer cells
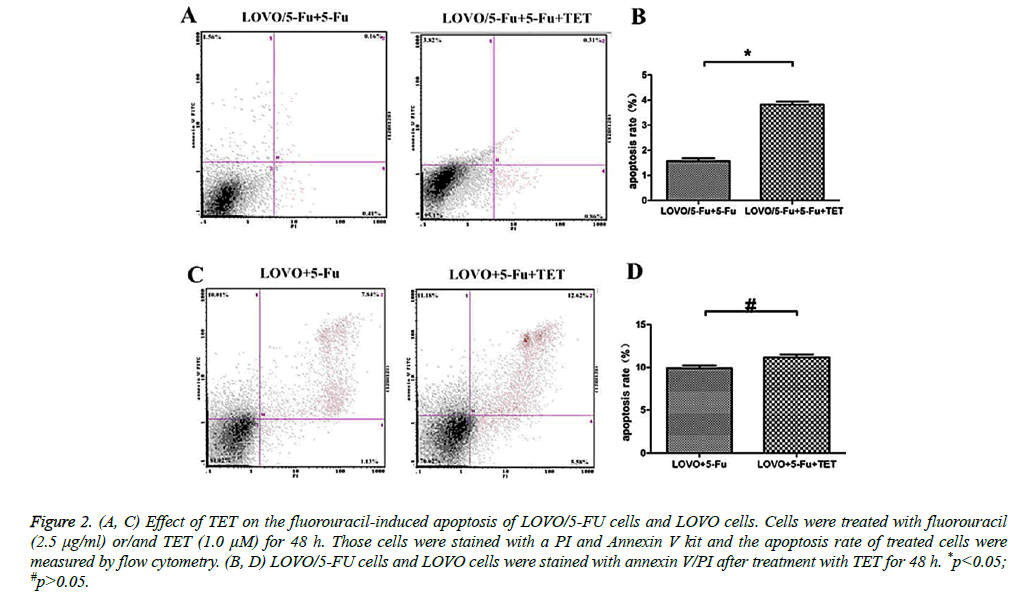
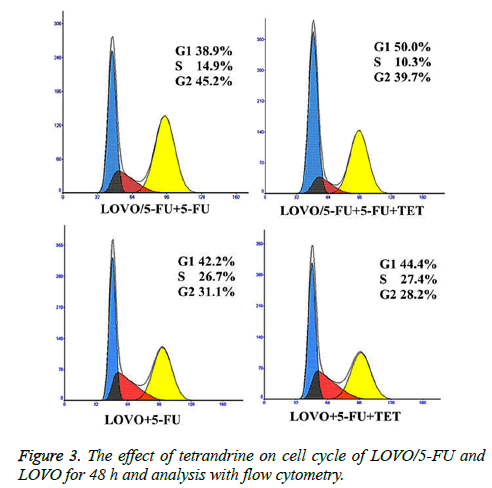
In order to examine whether TET could modulate the response of cells to fluorouracil, firstly the LOVO or LOVO/5-FU cells were incubated with 1.0 μM TET at a series concentrations of fluorouracil for 48 h. The IC50 and RI of fluorouracil in those two cell lines under the above treatment were evaluated by MTT colorimetric assay. Significantly, the sensitivity to fluorouracil of LOVO cells was much higher than that in LOVO/5-FU cells (Table 1), indicating that TET could effectively potentiate the response to 5-Fu of 5-Fu-resistant cancer cells. Next, we further analyzed the apoptosis rate of different LOVO cells under the treatment of 5-Fu with or without supplement of TET by flow cytometry analysis. Interestingly, LOVO/5-Fu cell line was resistance to 5-Fuinduced apoptosis, whereas the addition of TET greatly promoted the apoptosis rate of LOVO/5-Fu cells which were stimulated with 5-Fu (Figures 2A and 2B). At the same time, the effect of TET supplement was much less apparent on the LOVO cells (Figures 2B and 2C). We further investigated the impact of TET supplement on the proliferation of different LOVO cells and then we found that, with a combination of TET and 5-Fu, the number of LOVO/5-Fu cells in the S/G2 phases were greatly decreased when compared with those treated with 5-Fu solely (Figure 3). Meanwhile, it should be noted that the addition of TET did not change the response of LOVO cells to 5-Fu (Figure 3). Therefore, the treatment of TET potentiates the effect of 5-Fu on 5-Fu-resistant colon cancer cells and then reverses the drug resistance.
Figure 2: (A, C) Effect of TET on the fluorouracil-induced apoptosis of LOVO/5-FU cells and LOVO cells. Cells were treated with fluorouracil (2.5 μg/ml) or/and TET (1.0 μM) for 48 h. Those cells were stained with a PI and Annexin V kit and the apoptosis rate of treated cells were measured by flow cytometry. (B, D) LOVO/5-FU cells and LOVO cells were stained with annexin V/PI after treatment with TET for 48 h. *p<0.05; #p>0.05.
| IC50(μg/ml) | RI | |
|---|---|---|
| LOVO | 0.5 | 1 |
| LOVO + TET | 0.51 | 1.02 |
| LOVO/5-Fu | 6.91 | 13.78 |
| LOVO/5-Fu + TET | 4.15 | 8.28 |
Table 1. Effect of TET on the IC50 and RI of fluorouracil in LOVO/5- FU cells.
The reversal effect of TET on drug resistance is potentially dependent on P-glycoprotein
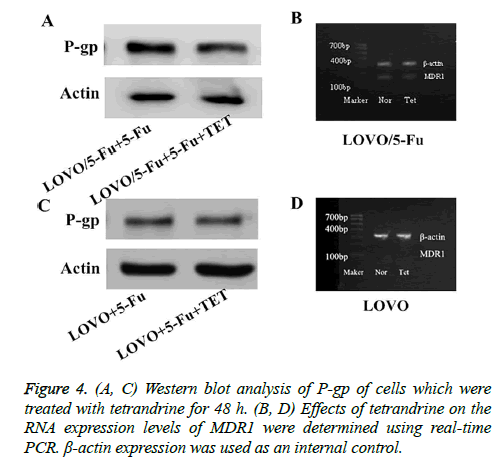
The P-gp gene encodes the drug eux pump protein Pglycoprotein, whose expression is correlated with the development of MDR. This is an important phenomenon during the drug resistance procession. We hypothesized that Pglycoprotein may also be involved in the 5-Fu-resistance of colon cancer cells and TET reversed the resistance mainly via modulating the expression level of P-gp. To test this hypothesis, we analyzed the mRNA level and protein level of P-gp. Interestingly, we found that the 5-Fu-resistant LOVO/5- FU had a greatly higher level of P-gp (MDR1), indicating that P-gp is involved in the drug resistance of this cancer cell variant (Figures 4A and 4C). Upon the treatment of TET, P-gp expression was decreased both at mRNA level and protein level in LOVO/5-FU cells but not in LOVO cells (Figures 4B and 4D). In conclusion, P-glycoprotein is a potential target for the regulation of 5-Fu resistance of colon cancer cells by TET. This suggests that the TET could be used as an additional drug which could be combined with the chemotherapeutical drug for colorectal cancer treatment.
Discussion
In the current cancer treatments, chemotherapy plays an essential role in the intervention of the most malignant tumors. However, only a small percentage of patients could get benefits, while the rest failed to respond to chemotherapy due to the intrinsic or acquired resistance. In particular, colon carcinoma cells could gain an MDR phenotype immediately after the initial chemotherapy. In that case, colon carcinoma patients are generally deemed refractory to cytotoxic drugs. Therefore, MDR is a major challenge for the achievement of chemotherapeutic success to colon carcinoma. Screening efficient sensitizers or reversal agents to MDR and combining them with chemotherapeutic drugs would be a promising strategy to overcome MDR and to provide successful management of colon carcinoma.
During the past decades, great efforts have been taken in developing and screening substances in order to reverse the MDR in cancer therapy. A few compounds, including verapamil, quinidine, cyclosporins and tamoxifen, have been shown to reverse the MDR activity in vitro. However, these compounds mostly are toxic and lead to disappointing the clinical results via inducing heart failure and immunosuppression [12]. Recently, several natural products derived from Chinese traditional medicine have been suggested as potential MDR reversal agents for cancers treatment including breast cancers [13], liver cancers [14], esophagus [10], cervix [15] and leukemia [16]. These observations indicate that Chinese traditional medicine could be a promising source of candidates for the novel MDR reversal agents.
Tetrandrine (TET), a bisbenzylisoquinoline alkaloid, is isolated from the root of the creeper Stephania tetrandra. It is reported as a non-selective antagonist of the calcium channel. Previous studies have shown that TET enhanced the tumor-toxic effect of vincristine via directly binding to MDR1 and promoting intracellular vincristine accumulation in oral cancer cells KBv200 [17]. It has also been revealed that TET exhibited even stronger activity to reverse drug resistance to adriamycin (ADM), vincristine (VCR), and etoposide in human epidermoid carcinoma cell line KB-MRP1 [18]. The derivatives of TET such as bromotetrandrine and H1, have also been found to be promising candidates as effective MDR reversing agent during the chemotherapy [19,20].
In this study, we firstly reported that TET played a reversal role on drug resistance to 5-FU in MDR human colon cancer, especially in the LOVO/5-FU cells lines. To test the sensitization of TET on LOVO/5-FU during the stimulation of 5-FU, the MTT assay were adopted respectively. Specifically, our results showed that the addition of TET significantly reduced the proliferation of LOVO/5-FU cells and promoted the apoptosis rate of these TET-treated cells. This inhibition effect of TET on cancer lines were caused via G1/S phage arrest. Thus the LOVO/5-FU cells could not be involved in the cell proliferation process. So what is the cellular mechanism of TET on the reversal eect of drug resistance? We know that Pglycoprotein (P-gp), a 170 kD plasma membrane glycoprotein, belongs to the superfamily of ATP-binding cassette transporters and actively pumps out a wide range of substance from cells, especially structurally and functionally diverse anticancer drugs, decreasing their intracellular accumulation [21-24]. By exploring the western blot study and q-PCR analysis, we found that TET probably modulates drug resistance by inhibiting P-glycoprotein gene expression. The inhibition of Pglycoprotein, the specific ATP-binding cassette transporter, in the present study is a cause for the reversal of P-gp by TET. Further studies are needed to thoroughly investigate the mechanisms of how TET modulating the expression of Pglycoprotein.
In conclusion, the present study demonstrated the reversal effect of TET on MDR in multidrug-resistant colon cancer cells at a relatively low concentration. TET, at a non-cytotoxic dose, inhibits the proliferation and arrests the cell cycle at the G1 phase, induces apoptosis, inhibits the drug efflux pump activity via down regulating of MDR1 in LOVO/5-FU cells. Thus, the TET may serve as a promising adjuvant for potentiating the effect of chemotherapy for colon cancers.
References
- Wong TW, Colombo G, Sonvico F. Pectin matrix as oral drug delivery vehicle for colon cancer treatment. AAPS PharmSciTech 2011; 12: 201-214.
- Dönmez Y, Akhmetova L, Iseri ÖD, Kars MD, Gündüz U. Effect of MDR modulators verapamil and promethazine on gene expression levels of MDR1 and MRP1 in doxorubicin-resistant MCF-7 cells. Cancer Chemother Pharmacol 2011; 67: 823-828.
- Fang JH, Tetrandine FY. Pharmacology and clinical usefulness. Chin Pharmaceutic J 1996; 31: 454-457.
- Banks DE, Cheng YH, Weber SL, Ma JK. Strategies for the treatment of pneumoconiosis. Occup Med 1993; 8: 205-232.
- Gao JL, Ji X, He TC, Zhang Q, He K, Zhao Y, Chen SH, Lv GY. Tetrandrine Suppresses Cancer Angiogenesis and Metastasis in 4T1 Tumor Bearing Mice. Evid Based Complement Alternat Med 2013; 2013: 265061.
- Liu W, Zhang J, Ying C, Wang Q, Yan C, Jingyue Y, Zhaocai Y, Yan X, Heng-Jun S, Lin J. Tetrandrine combined with gemcitabine and Cisplatin for patients with advanced non-small cell lung cancer improve efficacy. Int J Biomed Sci 2012; 8: 28-35.
- Yu VW, Ho WS. Tetrandrine inhibits hepatocellular carcinoma cell growth through the caspase pathway and G2/M phase. Oncol Rep 2013; 29: 2205-2210.
- Wu Z, Wang G, Xu S, Li Y, Tian Y, Niu H, Yuan F, Zhou F, Hao Z, Zheng Y, Li Q, Wang J. Effects of tetrandrine on glioma cell malignant phenotype via inhibition of ADAM17. Tumour Biol 2014; 35: 2205-2210.
- Shan QQ, Gong YP, Guo Y, Lin J, Zhou RQ, Yang X. Anti-tumor effect of tanshinone II A, tetrandrine, honokiol, curcumin, oridonin and paeonol on leukemia cell lines. Sichuan Da Xue Xue Bao Yi Xue Ban 2012; 43: 362-366.
- Wang TH, Wan JY, Gong X, Li HZ, Cheng Y. Tetrandrine enhances cytotoxicity of cisplatin in human drug-resistant esophageal squamous carcinoma cells by inhibition of multidrug resistance-associated protein 1. Oncol Rep 2012; 28: 1681-1686.
- Cui TY, Chen BA, Ding JH, Gao C, Cheng J, Bao W, Zhong YJ, Shan XY, Gao F, Xia GH, Schmitt A, Schmitt M. Inducing apoptosis and reversal effect of nilotinib in combination with tetrandrine on multidrug resistance of K562/A02 cell line. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2011; 19: 28-33.
- Robert J, Jarry C. Multidrug resistance reversal agents. J Med Chem 2003; 46: 4805-4817.
- Yang L, Wei DD, Chen Z, Wang JS, Kong LY. Reversal of multidrug resistance in human breast cancer cells by Curcuma wenyujin and Chrysanthemum indicum. Phytomedicine 2011; 18: 710-718.
- Wang XB, Wang SS, Zhang QF, Liu M, Li HL, Liu Y, Wang JN, Zheng F, Guo LY, Xiang JZ. Inhibition of tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug resistant human hepatocellular carcinoma cells. Oncol Rep 2010; 23: 211-215.
- Chanmahasathien W, Ampasavate C, Greger H, Limtrakul P. Stemona alkaloids, from traditional Thai medicine, increase chemosensitivity via P-glycoprotein-mediated multidrug resistance. Phytomedicine 2011; 18: 199-204.
- El-Readi MZ, Hamdan D, Farrag N, El-Shazly A, Wink M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur J Pharmacol 2010; 626: 139-145.
- Fu L, Liang Y, Deng L, Ding Y, Chen L, Ye Y, Yang X, Pan Q. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother Pharmacol 2004; 53: 349-356.
- Chen XS, Bao MH, Mei XD. Reversing multidrug resistance of epidermoid carcinoma drug-resistant cell line KB-MRP1 by tetrandrine. Ai Zheng 2007; 26: 846-850.
- Wei N, Sun H, Wang F, Liu G. H1, a novel derivative of tetrandrine reverse P-glycoprotein-mediated multidrug resistance by inhibiting transport function and expression of P-glycoprotein. Cancer Chemother Pharmacol 2011; 67: 1017-1025.
- Chen LM, Liang YJ, Zhang X, Su XD, Dai CL, Wang FP, Yan YY, Tao LY, Fu LW. Reversal of P-gp-mediated multidrug resistance by Bromotetrandrine in vivo is associated with enhanced accumulation of chemotherapeutical drug in tumor tissue. Anticancer Res 2009; 29: 4597-4604.
- Ling V. Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother Pharmacol 1997; 40: S3-S8.
- Li RJ, Zhang GS, Chen YH, Zhu JF, Lu QJ, Gong FJ, Kuang WY. Down-regulation of mitochondrial ATPase by hypermethylation mechanism in chronic myeloid leukemia is associated with multidrug resistance. Ann Oncol 2010; 21: 1506-1514.
- Liu Q, Shuhendler A, Cheng J, Rauth AM, O'Brien P, Wu XY. Cytotoxicity and mechanism of action of a new ROS-generating microsphere formulation for circumventing multidrug resistance in breast cancer cells. Breast Cancer Res Treat 2010; 121: 323-333.
- Xu WL, Shen HL, Ao ZF, Chen BA, Xia W, Gao F, Zhang YN. Combination of tetrandrine as a potential-reversing agent with daunorubicin, etoposide and cytarabine for the treatment of refractory and relapsed acute myelogenous leukemia. Leuk Res 2006; 30: 407-413.