Short Communication - Biomedical Research (2017) Volume 28, Issue 8
Technical protocol for infiltration of the humeral joint in a rat animal model
Claudia Andrea Vargas1,2*, Nicolas Ernesto Ottone2,3, Carlos Ivan Veuthey41Department of Physical Education, Sports and Recreation, Education School, Universidad de La Frontera, Temuco, Chile.
2Doctoral Program in Morphological Sciences, Medicine School, Universidad de La Frontera, Temuco Chile.
3Laboratory of Plastination and Anatomical Techniques, Research Centre in Dental Sciences (CICO), Dental School, Universidad de La Frontera, Temuco, Chile.
4Research Centre in Dental Sciences (CICO), Dental School, Universidad de La Frontera, Temuco, Chile.
- *Corresponding Author:
- Claudia Andrea Vargas
Department of Physical Education
Education School, Universidad de La Frontera, Chile
Accepted date: January 5, 2017
Abstract
Studies exist which confirm that the rat is the most appropriate model for assessing the glenohumeral (shoulder) joint and its pathologies, such as osteoarthritis and disease of the tendons of the rotator muscles in the joint. However there are no recorded studies of osteoarthritis induced in the Glenohumeral Joint (GJ) in rat models, nor is there a technical protocol for the correct approach to the joint. The object of this work was to establish a technical protocol, identifying anatomical reference points which would allow correct location of the penetration site for the articular cavity of the GJ followed by simulated infiltration. Four Sprague-Dawley rats were re-used for this protocol, respecting the animal ethics principles of refinement, reduction and replacement. The anatomical reference points were established and the joint was subsequently infiltrated with Indian ink. The success of the approach was corroborated by dissection of the joint, when the Indian ink could be sighted in the articular cavity. The rat's humerus was positioned in lateral rotation, using the sternoclavicular joint as a reference and following the lower margin of the clavicle until the coracoid process and the head of the humerus were reached. The mid-line of the humerus was calculated and the ink was injected below the intersection of the inferior margin of the clavicle and the mid-line of the humerus. Successful infiltration of the joint cavity requires knowledge of the morphology of the rat's GJ and the lateral rotation movement of the humerus, following the anatomical reference points identified. This technique can be applied for induction and treatment of osteoarthritis in models.
Keywords
Infiltration, Humeral joint, Rats, Technical protocol, Osteoarthritis.
Introduction
Osteoarthritis (OA) is the most common chronic rheumatic disease. Its manifestations are pain, deformity and functional disability, principally in joints with a large range of movement or which bear weight. Due to the progressive aging of the world's population and the increased prevalence of chronic degenerative diseases, osteoarthritis has become a public health problem which requires early diagnosis. Fifteen percent of the world's population aged over 60 suffer OA; the condition is thought to be ten to twelve times more frequent than rheumatoid arthritis [1]. It is characterized by cartilage erosion, chondrocyte proliferation and the formation of osteophytes at joint margins. The underlying bone is characterized by an increase in osteoclasts and osteoblast activity, causing alterations to the bone contours and the formation of subchondral cysts. Degenerative disease of the GJ is the third most frequent type, after osteoarthritis of the hip and knee [2].
Rats are widely used as animal models in experimental research and the testing of toxic drugs [3]. They can be used to understand the processes of cartilage lesions and to develop and evaluate treatments to prevent cartilage damage. They can also be used for histopathological examinations, and for assessing cartilage and meniscus degeneration and the development of oedemas in bone marrow after massive injury to the tendons of the rotator muscles of the GJ [4].
Previous studies have shown that the rat's glenohumeral joint imitates the anatomy and architecture of human bones and soft tissues (tendons and ligaments) [5,6]. There are many studies in rat models with induction of osteoarthritis by injection of sodium monoiodoacetate in various joints, mainly the knee [7] and the temporomandibular joint [8,9]; however no studies have been found in the literature of OA induction by MIA in rat glenohumeral joints.
The joint capsule is inserted on the superficial face of the glenoid labrum and on the surface of the glenoid cavity, extending in a medial direction to the scapular periosteum and the base of the coracoid apophysis. To the inferior it extends to the insertion of the tendon of the long head of the brachial triceps. The capsule surrounds the anatomical neck of the humerus. It is thin and lax, allowing a separation of up to 2 cm between the joint surfaces. The deep face of the joint capsule is lined by the synovial, which communicates to the anterior through the oval foramen with the subtendinous bursa of the subscapularis muscle. It also presents two recesses, one of which descends to the surgical neck of the humerus and the other to the intertubercular sulcus. The coracohumeral ligament extends from the base of the coracoid process to the superior margin of the greater and lesser tubercles of the humerus.
The object of this work was to establish a technical protocol to identify anatomical reference points which would allow correct location of the penetration site in the GJ cavity followed by simulated infiltration. This will allow future studies of induced osteoarthritis and treatment with injectable materials.
Materials and Methods
For this injection protocol four female Sprague-Dawley rats were used, obtained from an ethical source, weighing 200 to 250 g. Before any procedures were carried out, including imaging, they were anaesthetised with ketamine/xylazine by intraperitoneal injection (80 mg/kg and 10 mg/kg respectively). Euthanasia was carried out by overdose of the same anaesthetic.
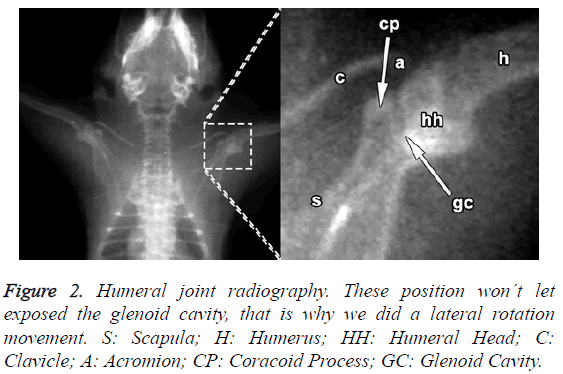
One of the rats was dissected for morphological study of the joint. The glenohumeral joint was dissected and extracted, and photographs were taken using a Leica S6D, stereo microscope, Leica MC120 HD microscope camera and Leica Application Suite EZ V3.0 image software. The other three were anaesthetised and studied by radiography, with the anatomical structures being identified at surface level; they were then killed by euthanasia and the Indian ink was injected. The success of the approach was corroborated by dissection and sighting of the Indian ink inside the articular capsule.
Description of the anatomy of the glenohumeral joint in rats (Figures 1A-1F)
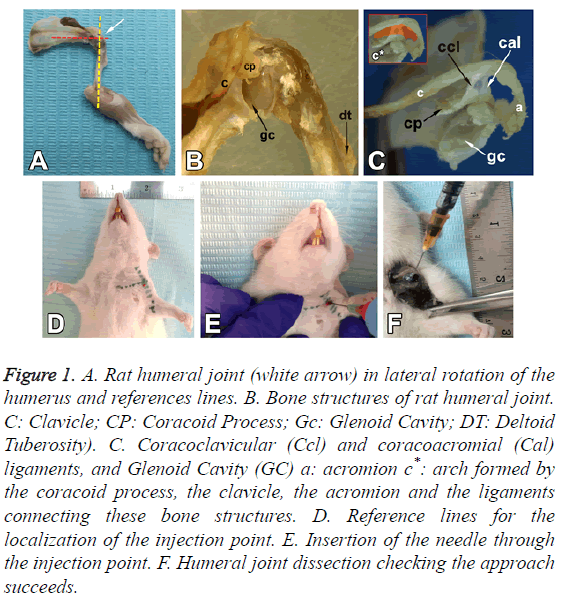
The rat has a prominent tendon of the supraspinatus muscle which is inserted in the greater tubercle of the humerus. As in humans, there is a relationship between the clavicle, the coracoid process, the acromion and the supraspinatus fossa. The tendon passes immediately below the arch formed by the coracoid process, the clavicle, the acromion and the ligaments connecting these bone structures (Figure 1C).
Figure 1: A. Rat humeral joint (white arrow) in lateral rotation of the humerus and references lines. B. Bone structures of rat humeral joint. C: Clavicle; CP: Coracoid Process; Gc: Glenoid Cavity; DT: Deltoid Tuberosity). C. Coracoclavicular (Ccl) and coracoacromial (Cal) ligaments, and Glenoid Cavity (GC) a: acromion c*: arch formed by the coracoid process, the clavicle, the acromion and the ligaments connecting these bone structures. D. Reference lines for the localization of the injection point. E. Insertion of the needle through the injection point. F. Humeral joint dissection checking the approach succeeds.
The coracoid process extends below the acromioclavicular joint, which is found above the glenohumeral joint, as far as the level at which the head of the humerus is articulated with the glenoid cavity of the scapula.
The rostral margin of the scapula in rats lacks the incisura present in humans; unlike humans, the rat's scapula is relatively longer to the dorso-ventral than to the cephalo-caudal (Figure 2).
The lateral end of the clavicle, the acromion and the coracoacromial ligament form an osteo-fibrous arch which limits the subacromial space (Figure 1C). This space contains the synovial bursa, the tendon of the supraspinatus muscle, a portion of the joint capsule and a part of the tendon of the long head of the biceps, which crosses the joint capsule.
The oval glenoid articular surface, which is enlarged by the labrum, is connected to the body of the scapula by a narrow, relatively long neck (Figure 1B).
The head of the humerus presents a hemispherical articular surface, one greater tubercle, one lesser and an intertubercular sulcus. A prominent deltoid tuberosity is observed laterally in the proximal part of the humerus (Figure 1B).
The glenohumeral joint in the rat is surrounded by a capsule whose external aspect is flattened by the superposition of the tendons of the scapulohumeral muscles.
Results
Anatomical reference points for the introduction of the needle and injection
The rat is placed in the dorsal decubitus position and the anatomical reference points are identified.
The sternoclavicular joint is identified by palpation (Figure 1D); the inferior margin of the clavicle is followed to lateral until the coracoid process is reached. The location of the latter between the lateral end of the clavicle and the head of the humerus, below the acromioclavicular joint, allows the articular cavity of the GJ to be identified. A lateral rotation is applied to the humerus to leave the deltoid tuberosity to lateral. A reference line is drawn, passing through the anatomical reference structures mentioned above, to intersect perpendicularly the mid-line of the humerus in the lateral rotation position, making the articular cavity more accessible for injection (Figure 1A).
Discussion
OA is classified into primary (idiopathic) and secondary [10]. In advanced stages of massive damage to the rotator cuff, progressive migration of the humerus head to the superior occurs, reducing the subacromial space and finally provoking degenerative changes in the GJ and in some cases osteoarthritis [11]. Primary OA of the glenohumeral joint is more prevalent than secondary [12]. Clinically, the altered mechanical load resulting from articular instability has been invoked as the primary initiation mechanism for osteoarthritis. Signs of anomalies in the bone marrow are frequent [4]. The most common mechanism for inducing OA in the glenohumeral joint of rats is tenotomy [13,14], meaning that the rats must be subjected to surgery. The advantage of intra-articular injection is that it is a simple procedure, in which the progression and gravity of the articular lesions can easily be modulated by modifying the concentration of the substances. Chondrocyte metabolism inhibitor (sodium Monoiodoacetate, MIA), has been reported to induce interruption of glycolysis and subsequently cell death and chondrocyte loss [7]. When it was used in rats, the model reproduced cartilage lesions with loss of proteoglycans from the matrix and functional deterioration of the joint similar to the effects of osteoarthritis in humans. The cartilage lesions are characterized by chondrocyte necrosis, cloning of cells (chondrocytes), fibrillation, loss of proteoglycan staining in the matrix and erosion with exposure of the subchondral bone. Bone lesions are reported, including remodelling and sclerosis of the subchondral bone with formation of osteophytes [7].
The anatomical characteristics of the rat's glenohumeral joint must be remembered for the needle to be introduced successfully, following the anatomical reference points of the sternoclavicular joint, and the inferior margin of the clavicle to the coracoid process located between the lateral end of the clavicle and the head of the humerus. A reference line is drawn which intersects perpendicularly the mid-line of the rat's humerus positioned in lateral rotation; this facilitates the needle's access to the articular capsule of the joint, at an angle of 90º and 1 mm below the coracoid process.
To ensure the success of the procedure, we recommend carrying out a test injection following the protocol indicated, injecting Indian ink, followed by dissection of the rat's glenohumeral joint to check that the needle did in fact enter the articular capsule.
References
- Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nat Rev Drug Discov 2005; 4: 331-344.
- Gutiérrez V, Ekdahl M. Gleno-humeral Osteoarthritis. Rev Med Clin Condes 2014; 25: 732-737.
- Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact 2001; 1: 363-376.
- Link TM, Li X. Bone marrow changes in osteoarthritis. Semin Musculoskelet Radiol 2011; 15: 238-246.
- Norlin R, Hoe-Hansen C, Oquist G, Hildebrand C. Shoulder region of the rat: anatomy and fiber composition of some suprascapular nerve branches. Anat Rec 1994; 239: 332-342.
- Soslowsky LJ, Carpenter JE, DeBano CM, Banerji I, Moalli MR. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg 1996; 5: 383-392.
- Guingamp C, Gegout-Pottie P, Philippe L, Terlain B, Netter P, Gillet P. Mono-iodoacetate-induced experimental osteoarthritis: a dose-response study of loss of mobility, morphology, and biochemistry. Arthritis Rheum 1997; 40:1670-1679.
- Cledes G, Felizardo R, Foucart JM, Carpentier P. Validation of a chemical osteoarthritis model in rabbit temporomandibular joint: a compliment to biomechanical models. Int J Oral Maxillofac Surg 2006; 35:1026-1033.
- Wang XD, Kou XX, He DQ, Zeng MM, Meng Z, Bi RY, Liu Y, Zhang JN, Gan YH, Zhou YH. Progression of cartilage degradation, bone resorption and pain in rat temporomandibular joint osteoarthritis induced by injection of iodoacetate. PLoS One 2012; 7: e45036.
- Lavalle MC. Osteoartritis. MExico DF Universidad Nacional Autónoma de MExico, 2010.
- Gutiérrez V, Ekdahl M. Gleno-humeral Osteoarthritis. Rev Med Clin Condes 2014; 25: 732-737.
- Millett PJ, Gobezie R, Boykin RE. Shoulder osteoarthritis: diagnosis and management. Am Fam Physician 2008; 78: 605-611.
- Galatz LM, Silva MJ, Rothermich SY, Zaegel MA, Havlioglu N. Nicotine delays tendon-to-bone healing in a rat shoulder model. J Bone Joint Surg Am 2006; 88: 2027-2034.
- Kramer EJ, Bodendorfer BM, Laron D, Wong J, Kim HT. Evaluation of cartilage degeneration in a rat model of rotator cuff tear arthropathy. J Shoulder Elbow Surg 2013; 22: 1702-1709.