Research Article - Biomedical Research (2017) Volume 28, Issue 18
T cell subsets and cytokines are increased in the bronchoalveolar lavage fluid in children with pneumonia
Hui Xu1#, Li Leng2#, Min Chen1, Bi Wang3, Yongfeng Sun1, Qi Wang1 and Rong Jin1*
1Department of Paediatrics, Maternal and Child Health Hospital of Guiyang City, Guiyang, Guizhou, PR China
2Department of Clinical Teaching, Maternal and Child Health Hospital of Guiyang City, Guiyang, Guizhou, PR China
3Department of Prepotency, Maternal and Child Health Hospital of Guiyang City, Guiyang, Guizhou, PR China
#These authors have equally contributed to this study.
- *Corresponding Author:
- Rong Jin
Department of Paediatrics
Maternal and Child Health Hospital of Guiyang City
Guiyang, Guizhou, PR China
Accepted on August 16, 2017
Abstract
Objective: Pneumonia is a common disease in infants that has been a major cause of death in children worldwide. Although studies have suggested that T cell subsets and cytokines in the Bronchoalveolar Lavage Fluid (BALF) may reflect the severity of pneumonia, the exact connection remains un-clarified. This study aimed to elucidate the changes of T cell subsets and cytokines in BALF in paediatric pneumonia.
Patients and Methods: A total of 90 cases of pneumonia children who were examined in our hospital between January 2015 and January 2016 were selected in this study. The patients were divided into mild-to-moderate (n=36) and severe pneumonia group (n=54). Thirty children without any lung diseases were used as controls. The bronchial secretion was collected and analyzed by flow cytometry to determine the expression of surface activation marker of CD3+ T cell subpopulation (CD69, CD25 and HLA-DR). The expression of IL-6, IL-17 and HMGB1 was measured by ELISA.
Results: The expression of CD69, CD25 and HLA-DR in BALF in patients was significantly higher compared with controls (P=0.033). The level of IL-6, IL-17 and HMGB1 in patients was also significantly higher than that in control (P=0.026). The expression of CD69, CD25 and HLA-DR as well as IL-6, IL-17 and HMGB1 in severe pneumonia group was significantly higher compared with mild-tomoderate pneumonia group (P <0.05).
Conclusions: The significant increase in the surface activation markers and cytokines in BALF suggested the immune dysfunction in pneumonia patients. The positive correlation between cytokine expression and the severity of pneumonia indicated their role as biomarkers in immunotherapy.
Keywords
Pneumonia, Bronchoalveolar lavage fluid (BALF), T cells, Cytokines.
Introduction
Pneumonia is a common disease that has been a major health threat to children worldwide. The disease results in approximately 200 million deaths of children aged younger than 5 per year. The incidence rate of severe pneumonia in young children less than 3 y old is around 10-15%, ranking first among all paediatric critical diseases [1-3]. The researches on the pathogens and other causes of pulmonary infection, as well as immune pathological mechanism have received extensive attention.
T lymphocytes play an important role in the regulation of the body’s immune system. T lymphocytes are generally analyzed by the detection of T cell surface antigen. In this study, the T cell common antigens CD3+CD69+, CD3+CD25+ and CD3+HLA-DR+ were selected for the analysis of T cell subsets [4-7]. IL-6 is a pro-inflammatory cytokine produced by T cells and monocytes. It has been suggested that the elevated expression of IL-6 may be used as an indicator for infection [8]. IL-17 is a novel pro-inflammatory cytokine secreted by Th17 cells that is crucial for the regulation of inflammation and autoimmune diseases [9]. High Mobility Group protein (HMGB1) is a highly conserved non-histone chromosomal binding protein that is widely present in animal tissues and cells. The serum HMGB1 level is extremely low in healthy people, but is markedly increased in patients with various diseases such as sepsis and chronic hepatitis B, suggesting that HMGB1 plays an important role in the development of inflammation [9-11]. For instance, the HMGB1 level in patients with haemorrhagic shock is increased in a timedependent manner and peaks at 72 h after the onset. The HMGB1 level is gradually recovered to normal level as the clinical symptoms are alleviated. The high expression of HMGB1 in several cancers further suggests its involvement in the pathogenesis of multiple diseases [12,13]. Extracellular HMGB1 is an essential inflammatory cytokine, and its expression level is associated with the severity of diseases [14-16]. Nevertheless, the pathological significance of HMGB1 in paediatric pneumonia remains unclear. In this study, the HMGB1 expression in paediatric pneumonia and its connection with the disease severity was investigated.
The changes in T cell subsets and cytokines in paediatric pneumonia have been previously reported. These studies, however, are generally conducted on blood or sputum samples. There are currently very few studies that investigated the T cell subsets and cytokines in the Bronchoalveolar Lavage Fluid (BALF) [17]. BALF is directly collected in the bronchial and alveolar lesions in patients, and therefore can more accurately reflect the condition of the disease when compared with blood and sputum samples. In the current study, the expression of T cell surface antigens and cytokines in BALF in paediatric pneumonia was detected to elucidate their association with the severity of the disease. The study will shed lights on the immune mechanisms of severe pneumonia and provide new biomarkers for the treatment and prognosis of the disease.
Materials and Methods
Subjects
A total of 90 cases of pneumonia children who were examined in Maternal and Child Health Hospital of Guiyang city between January 2015 and January 2016 were selected in this study, including 34 boys and 56 girls with a mean age of 3.8 ± 0.8 y. The patients were divided into mild-to-moderate (n=36) and severe pneumonia group (n=54). Thirty children without any lung diseases were used as controls, including 12 boys and 18 girls with a mean age of 3.6 ± 1.1 y. The subjects had none of the following diseases: blood diseases, central nervous system diseases, tuberculosis and immunodeficiency diseases such as cancer.
This study has been pre-approved by the ethical committee of Maternal and Child Health Hospital of Guiyang city. All of the samples were confirmed by family members and signed the consent form.
Main reagents
HMGB1 ELISA kit was purchased from Chuangxiang Biotech. (Shanghai, China). Flow cytometry kit was purchased from Immunotech (Marseille, France). IL-6 ELISA kit was purchased from Baiaolaibo Biotech. (Beijing, China). Cytokine detection kit was purchased from Beacon Inc. PBS buffer was purchased from Beyotime Biotech. Flow cytometry was purchased from Thermo Fisher Scientific. Plate reader was purchased from BD Biosciences. Centrifuge was purchased from Takara.
Extraction of BALF
All children with pneumonia were examined by chest CT and samples were collected from the largest area of infiltration in the lung. Samples were collected from the right middle lobe in normal control group. Briefly, a bronchoscope was inserted into the sampling site, and 5 ml of 37°C preheated saline was slow pumped through the bronchoscope using a syringe. The sampling site was slowly rinsed 2-3 times. The BALF was then slowly extracted and filtered through sterile gauze. All BALF samples were centrifuged at 1500 rpm/min at 4°C. The supernatant was transferred to a new EP tube and stored at -80°C until use.
Flow cytometry analyses of T cell surface activation molecule
Samples were completely thawed on ice. Aliquots of samples (500 μl) were added into the sample tube. CD3+ FITC/CD69+ PE, CD3+ FITC/CD25+ PE or CD3+ FITC/HLA-DR+ PE was then added to the appropriate tube and mixed for 10 s with an oscillator. The mixture was allowed to react for 15 min in dark and incubated at 37°C for additional 20 min. Cells were collected by centrifugation at 3000 rpm/min for 5 min and resuspended in 200 μl of PBS. The cell suspension was examined by flow cytometer to detect the expression of T cell surface activation molecules.
ELISA analyses of IL-6, IL-17 and HMGB1 level
The level of IL-6, IL-17 and HMGB1 in BALF was determined by ELISA. Briefly, aliquots of samples (100 μl) were added into the appropriate ELISA plate and incubated at room temperature for 2 h. Each well was rinsed 5 times with 300 μl of washing solution. Antibody solution (100 μl) was added into each well and the plate was incubated at room temperature for 1 h. Each well was rinsed 5 times with 300 μl of washing solution and incubated in 100 μl of enzyme conjugate working solution in dark for 30 min. The reaction was terminated by adding 50 μl of stop solution. The OD was detected by a microplate reader at the wavelength of 450 nm. Standard samples and blank control were added into each plate. Three replicates were prepared for each sample and the mean OD value was calculated as the IL-6, IL-17 and HMGB1 level.
Statistical analysis
All data were expressed as mean ± standard deviation and analyzed using SPSS 16.0 (IBM SPSS, Chicago, IL, USA). Difference among groups was compared by ANOVA. P values smaller than 0.05 are considered statistically significant.
Results
Expression of T cell surface activation molecules
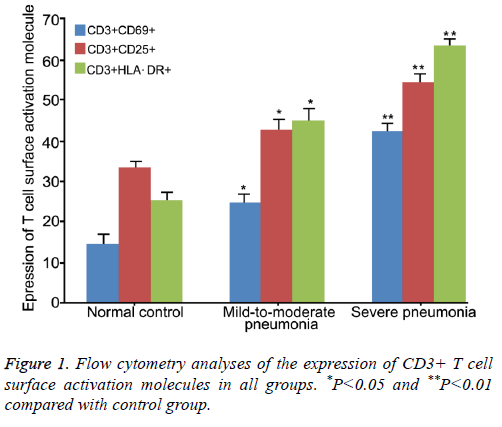
Flow cytometry analyses showed that the expression of CD3+ CD69+, CD3+ CD25+ and CD3+ HLA-DR+ in severe pneumonia group (42.32 ± 4.87, 54.36 ± 4.35 and 63.66 ± 5.63, respectively) was significantly higher compared with mild-to-moderate group (24.61 ± 3.25, 42.82 ± 3.73 and 44.92 ± 5.42, respectively, P<0.05, Table 1).
| Group | Normal control | Mild-to-moderate pneumonia | Severe pneumonia |
|---|---|---|---|
| n | 30 | 54 | 36 |
| CD3+CD69+ (%) | 14.66 ± 2.65 | 24.61 ± 3.25*∆ | 42.32 ± 4.87* |
| CD3+CD25+ (%) | 33.21 ± 3.21 | 42.82 ± 3.73*∆ | 54.36 ± 4.35* |
| CD3+HLA-DR+ (%) | 25.27 ± 3.36 | 44.92 ± 5.42*∆ | 63.66 ± 5.63* |
*P<0.05 compared with control group; ∆P<0.05 compared with severe pneumonia group.
Table 1. Flow cytometry analyses of the expression of CD3+ T cell surface activation molecules in all groups.
The expression of the three T-cell surface antigens in both groups was significantly higher compared with control group (14.66 ± 2.65, 33.21 ± 3.21 and 25.27 ± 3.36, respectively, P<0.05 or 0.01). These results suggested that the CD3+ T cell subsets were markedly increased during the occurrence of pneumonia, and was positively associated with the disease severity (Figure 1).
Comparison of IL-6, IL-17 and HMGB1 level in BALF
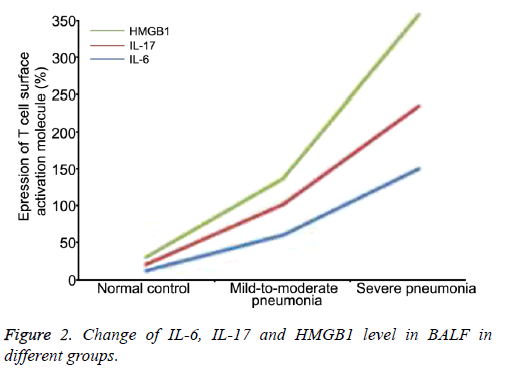
ELISA results showed that the IL-6, IL-17 and HMGB1 level in both pneumonia groups was significantly higher than that in control group (P<0.05 or 0.01, Table 2).
| Group | IL-6 (ng/ml) | IL-17 (pg/ml) | HMGB1 (ng/ml) |
|---|---|---|---|
| Normal control | 12.32 ± 2.13 | 8.61 ± 2.25 | 10.12 ± 2.13 |
| Mild-to-moderate pneumonia | 60.21 ± 2.31*∆ | 42.82 ± 3.73*∆ | 34.35 ± 5.25*∆ |
| Severe pneumonia | 151.2 4 ± 3.36** | 84.92 ± 6.48** | 123.65 ± 15.63** |
*P<0.05 and **P<0.01 compared with control group; ∆P<0.05 compared with severe pneumonia group.
Table 2. Comparison of IL-6, IL-17 and HMGB1 level in BALF in different groups by ELISA.
The severe pneumonia group exhibited significantly higher IL-6, IL-17 and HMGB1 level compared with mild-tomoderate pneumonia group (P<0.05), suggesting that the level of these cytokines was closely associated with the severity of the disease (Figure 2).
Discussion
Pneumonia may be caused by infection of a variety of pathogens such as bacteria and viruses, and therefore should be symptomatically treated. Although the clinical treatment of pneumonia has received increased attention, and several new drugs and antibiotics for pneumonia has been introduced in recent years, the incidence and mobility rate of severe paediatric pneumonia is gradually increasing, suggesting that the therapeutic effect of antibiotics is relatively limited for the disease [18]. In some cases, treatment failure may occur, leading to subsequent functional dysfunction of the lung, and even death of the patient. Therefore, researches on the etiology, pathogens and immune pathological mechanism of pneumonia have received extensive concerns. T cells are known to play an essential role in the regulation of the body’s immune system. Numerous studies have been previously performed on the T cell subsets and cytokines in blood or sputum samples in severe paediatric pneumonia [19]. Nevertheless, there are currently only very few studies on the BALF and the findings remain controversy. The change in T-cell subset in pneumonia patients and its connection with the disease severity needs further investigation.
As early inflammatory cytokines, IL-6 and IL-17 can clearly indicate the occurrence and development of inflammation [20]. In this study, the expression of T cell surface antigens and cytokines in BALF in mild-to-moderate, severe paediatric pneumonia and control group was compared. It was found that the expression of T cell surface antigens, IL-6, IL-17 and HMGB1 in both pneumonia groups was significantly higher than that in control group (P<0.05 or 0.01). Moreover, the severe pneumonia group exhibited significantly higher expression compared with mild-to-moderate pneumonia group (P<0.05), suggesting that the elevated expression of level of T cell surface antigen can be used as an indicator for the diagnosis of pneumonia. It is worth noting that the expression of HMGB1 was positively correlated with the severity of pneumonia, which might be attributed to its stimulatory effect on the expression of early inflammatory factors as a late inflammatory factor.
Conclusion
In this study, we investigated the changes of T cell subsets and cytokines in BALF in paediatric pneumonia, and found that the expression of T cell surface antigens, IL-6, IL-17 and HMGB1 in both pneumonia groups was significantly higher than that in control group. The expression in severe pneumonia group was significantly higher than that in mild-to-moderate pneumonia group, revealing the positive association between the expression of T cell subsets and cytokines and the disease severity. The current study has not only confirmed the significance of BALF in the determination of the condition of pneumonia, but also provided a theoretical basis for the identification of new biomarkers for the treatment and prognosis of the disease.
Acknowledgements
This project supported by the Guiyang Science and Technology Development Funds (20151001-She13) and Guizhou Science and Technology Development Funds ((LH) 20157029).
References
- Michelow IC, Katz K, McCracken GH, Hardy RD. Systemic cytokine profile in children with community-acquired pneumonia. Pediatr Pulmonol 2007; 42: 640-645.
- Ling C, Qian S, Wang Q, Zeng J, Jia X, Liu J, Li Z. Pneumocystis pneumonia in non-HIV children: A 10-year retrospective study. Clin Respir J 2016.
- Guo WL, Wang J, Zhu LY, Hao CL. Differentiation between mycoplasma and viral community-acquired pneumonia in children with lobe or multi foci infiltration: a retrospective case study. Bio Med J 2015; 5: e006766.
- Esposito S, Garziano M, Rainone V, Trabattoni D, Biasin M, Senatore L, Marchisio P, Rossi M, Principi N, Clerici M. Immunomodulatory activity of pidotimod administered with standard antibiotic therapy in children hospitalized for community-acquired pneumonia. J Transl Med 2015; 13: 288.
- Williams DJ, Hall M, Auger KA, Tieder JS, Jerardi KE, Queen MA, Statile AM, Myers AL, Shah SS. Association of white blood cell count and c-reactive protein with outcomes in children hospitalized for community-acquired pneumonia. Pediatr Infect Dis J 2015; 34: 792-793.
- Guo L, Liu F, Lu MP, Zheng Q, Chen ZM. Increased T cell activation in BALF from children with Mycoplasma pneumoniae pneumonia. Pediatr Pulmonol 2015; 50: 814-819.
- Wang M, Wang Y, Yan Y, Zhu C, Huang L, Shao X, Xu J, Zhu H, Sun X, Ji W, Chen Z. Clinical and laboratory profiles of refractory Mycoplasma pneumoniae pneumonia in children. Int J Infect Dis 2014; 29: 18-23.
- Guo H, He Z, Li M, Wang T, Zhang L. Imbalance of peripheral blood Th17 and Treg responses in children with refractory Mycoplasma pneumoniae pneumonia. J Infect Chemother 2016; 22: 162-166.
- Zhao MQ, Wang LH, Lian GW, Xie JH, Guo M, Zhang YY, Zhu B. The serum value of NO and IL-17 were increased in children with influenza a viral pneumonia. Clin Lab 2015; 61: 1415-1421.
- Zhou WF, Chen Q, Jin MF, Ji ZH, Zhang MZ, Li HM, Liu FJ, Ji W. The diagnostic accuracy of high-mobility group box 1 protein and twelve other markers in discriminating bacterial, viral and co-infected bronchial pneumonia in Han children. Microbiol Immunol 2011; 55: 279-288.
- Ito Y, Torii Y, Ohta R, Imai M, Hara S, Kawano Y, Matsubayashi T, Inui A, Yoshikawa T, Nishimura N, Ozaki T, Morishima T, Kimura H. Increased levels of cytokines and high-mobility group box 1 are associated with the development of severe pneumonia, but not acute encephalopathy, in 2009 H1N1 influenza-infected children. Cytokine 2011; 56: 180-187.
- Fan Q, Gu T, Li P, Yan P, Chen D, Han B. Roles of T-cell immunoglobulin and Mucin domain genes and toll-like receptors in wheezy children with mycoplasma pneumoniae pneumonia. Heart Lung Circ 2016.
- Yanik GA, Grupp SA, Pulsipher MA, Levine JE, Schultz KR, Wall DA, Langholz B, Dvorak CC, Alangaden K, Goyal RK, White ES, Collura JM, Skeens MA, Eid S, Pierce EM, Cooke KR. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome: A joint paediatric blood and marrow transplant consortium and children's oncology group study (ASCT0521). Biol Blood Marrow Transplant 2015; 21: 67-73.
- Rhedin S, Lindstrand A, Hjelmgren A, Ryd-Rinder M, Öhrmalm L, Tolfvenstam T, Örtqvist Å, Rotzén-Östlund M, Zweygberg-Wirgart B, Henriques-Normark B, Broliden K, Naucler P. Respiratory viruses associated with community-acquired pneumonia in children: matched case-control study. Thorax 2015; 70: 847-853.
- Erdman LK, D'Acremont V, Hayford K, Rajwans N, Kilowoko M, Kyungu E, Hongoa P, Alamo L, Streiner DL, Genton B, Kain KC. Biomarkers of host response predict primary end-point radiological pneumonia in Tanzanian children with clinical pneumonia: A prospective cohort study. PloS One 2015; 10: e0137592.
- Parikh K, Hall M, Mittal V, Montalbano A, Mussman GM, Morse RB, Hain P, Wilson KM, Shah SS. Establishing benchmarks for the hospitalized care of children with asthma, bronchiolitis, and pneumonia. Pediatrics 2014; 134: 555-562.
- Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J 2014; 33: 136-142.
- Caselli D, Petris MG, Rondelli R, Carraro F, Colombini A, Muggeo P, Ziino O, Melchionda F, Russo G, Pierani P, Soncini E, DeSantis R, Zanazzo G, Barone A, Cesaro S, Cellini M, Mura R, Milano GM, Meazza C, Cicalese MP, Tropia S, De Masi S, Castagnola E, Aricò M. Infectious diseases working group of the Associazione Italiana Ematologia Oncologia Pediatrica: Single-day trimethoprim/sulfamethoxazole prophylaxis for Pneumocystis pneumonia in children with cancer. J Pediatr 2014; 164: 389-392.
- Wrotek A, Jackowska T. Hyponatremia in children hospitalized due to pneumonia. Adv Exp Med Biol 2013; 788: 103-108.
- Chang TP, Kriengsoontorkij W, Chan LS, Wang VJ. Clinical factors and incidence of acute chest syndrome or pneumonia among children with sickle cell disease presenting with a fever: a 17-year review. Pediatr Emerg Care 2013; 29: 781-786.