Research Article - Biomedical Research (2018) Volume 29, Issue 5
Systemic inflammation response index (SIRI) as a predictor for predicting inflammatory bowel disease (IBD) severity
Ting Yu, Qian Yu*, Xilong Ou and Dazhong Cao
Department of Gastroenterology, Zhongda Hospital, School of Medicine, Southeast University, Nanjing, Jiangsu, PR China
- *Corresponding Author:
- Qian Yu
Department of Gastroenterology
Zhongda Hospital
School of Medicine
Southeast University, PR China
Accepted date: November 29, 2017
DOI: 10.4066/biomedicalresearch.29-17-3202
Visit for more related articles at Biomedical ResearchAbstract
Peripheral venous blood markers may reflect the systematic inflammatory response condition. Various indices have been used to evaluate Inflammatory Bowel Disease (IBD), however, there is no ideal maker to assess the IBD activity and severity. We aim to investigate the potential value of Systemic Inflammation Response Index (SIRI) in IBD patients, and use it to predict the disease activity and severity. We designed a retrospective study to evaluate the SIRI in estimating disease severity in patients with IBD. We enrolled 210 patients with IBD, included 110 cases of Ulcerative Colitis (UC) and 100 cases of Crohn’s Disease (CD) in our hospital, between May 2011 and October 2014. The blood samples were collected from the IBD patients before any treatment of antibiotics, steroids and surgery. The categorical variables were provided with frequencies and percentages (%), and using the Chi-square test. The univariate and multivariate logistic regression model analysis were used to determine the demographic and clinicopathological features with active IBD. The optimal cut-off value of SIRI by Receiver Operating Characteristic curve (ROC) was 0.86 × 109/L, and patients were stratified into 2 groups: a low SIRI group (SIRI<0.86 × 109/L) and a high SIRI group (SIRI ≥ 0.86 × 109/L). A high SIRI was significantly correlated with active IBD (P=0.019), CRP (P=0.002), ESR (P<0.001), NLR (P<0.001) and MLR (P<0.001). The active IBD was statistically significant between the two groups (P=0.019). Compared with the inactive IBD, the mild active and moderate active IBD were not statistically significant (P=0.907, 0.137), however, the severe active IBD was statistically significant (P<0.001). There was statistically significant of the different severity of active IBD between low SIRI group and high SIRI group (P=0.003). Multivariate analysis indicated that the CRP, ESR and SIRI were independently associated with active IBD. SIRI is cost-effective and convenient indicator in IBD patients and may be a new promising marker of the disease severity in IBD. High SIRI may help the doctors to make decisions for patients with active IBD.
Keywords
Systemic inflammation response index, Inflammatory bowel disease, Ulcerative colitis, Crohn’s disease, Creactive protein, Erythrocyte sedimentation rate
Introduction
Inflammatory Bowel Disease (IBD) is one of the common disease with dysregulation inflammation in the gastrointestinal tract, and severely influencing the physical and mental health of people [1]. IBD is a complex disorder with immune system, microbiota, genetic and environmental influences, and includes Ulcerative Colitis (UC) and Crohn's Disease (CD), which are associated with aberrant immune [2,3]. Intestinal neoplasms and inflammation are determinate relationships with each other, and acknowledged as a long term complication of IBD [4]. UC is a chronic and relapsing inflammatory disease, and causes continuous inflammation of the colorectal mucosa [5]. CD is a chronic and remitting inflammatory disease with an unknown etiology, and involves many factors, such as immune mediated response, genetic susceptibility factor and environmental factor [6].
One study of Western population-based has indicated that the risk of Colorectal Cancer (CRC) with IBD was higher than without IBD, and the disease still has a poor prognosis and leads hundreds of thousands of deaths annually [7]. However, with the better IBD management, the CRC with IBD was decreasing by effectively and efficiently suppressing intestinal inflammation [8]. The IBD incidence rate in Western countries is higher than the rates in Asian countries, but the incidence of IBD in Asian countries has been rapidly rising and the burden of this disease has been increasing, too [9]. Although some techniques have been used for diagnose, they are invasive techniques, such as histopathologic, endoscopic and so forth, and often time-consuming and expensive. Peripheral blood tests at the time of diagnosis and treatment can reflect inflammatory conditions within the IBD, and is also an ideal non-invasive test [10]. Evaluation of peripheral blood parameters including white blood cell (W), Neutrophil (N), Lymphocyte (L), Monocyte (M), Platelet (P) counts, as well as SIRI (N × M/L), which are indicators of systematic inflammatory response condition, and have been widely proposed as a useful biomarker of systemic inflammation responses [11-14].
As far as I’m concerned, an integrated indicator based on peripheral neutrophil, monocyte, and lymphocyte counts has not yet been reported in IBD, and might be better able to reflect the balance of host inflammatory and immune status. The aim of present study is to predict the severity in peripheral blood samples from patients with IBD.
Materials and Methods
Patient selection
We enrolled 210 patients with IBD, included 110 cases of UC and 100 cases of CD in our hospital, between May 2011 and October 2014. All patients with IBD were confirmed in accordance with pathological evidence, standard clinical, radiological, endoscopic and laboratory. The present study was conducted under the understanding and written consent of each patient and kin. This study was approved by the ethics committee of our hospital and in accordance with the ethical standards of the Declaration of Helsinki and its later ethical standards. Informed consent was obtained from all individual participants. The clinical and demographic data of patients were extracted from the patients’ medical records. Inclusion criteria included: (1) patients with IBD were confirmed in accordance with endoscopic evaluation with tissue histology; (2) aged ≥ 18 y; (3) no previous treatment, such as antibiotics, steroids and immunosuppressive agents. Exclusion criteria included: (1) patients with any chronic systemic disease, such as diabetes mellitus, hypertension, atherosclerotic heart diseases, hyperthyroidism, and adrenal insufficiency; (2) with any other malignancy or autoimmune disease; (3) patients with serious complications, such as infections, intestinal tuberculosis and ischemia.
Disease activity or severity
UC activity was evaluated by using Simple Clinical Colitis Activity Index (SCCAI), where SCCAI scores under 3 were considered inactive, SCCAI scores above 3 was considered active. And SCCAI scores of 3-5 were considered mild, SCCAI scores of 6-11 were considered moderate, SCCAI scores of above 12 were considered severe [15]. Crohn’s disease severity was defined by CD Activity Index (CDAI), where CDAI scores under 150 were considered inactive, CDAI scores above 150 were considered active [16]. Moreover, the activity of CD was evaluated by defined as mild (CDAI scores of 150-220), moderate (CDAI scores of 221-450) and severe (CDAI scores of above 450).
Blood sample
The peripheral venous blood samples were collected from the IBD patients before any treatment of antibiotics, steroids and surgery. These blood samples were collected into a sterile Ethylenediaminetetraacetic Acid (EDTA) tube. SIRI was defined as N × M/L, where N, M, and L are the pretreatment peripheral neutrophil, monocyte, and lymphocyte counts, respectively.
Statistical analysis
All statistical analyses were performing using Statistical Package for Social Sciences (SPSS) 19.0 (SPSS Inc., Chicago, IL, USA) and GraphPad prism software (version 5.0; GraphPad Inc., La Jolla, CA, USA). Receiver Operating Characteristic (ROC) curve analysis was used to decide the optimal cut-off values of SIRI and other inflammatory markers. The categorical variables were provided with frequencies and percentages (%), and the Chi-square test was used to compare these categorical variables. The univariate and multivariate logistic regression model analysis were used to determine the demographic and clinicopathological features with active IBD. Results were reported as Hazard Ratios (HR) and 95% Confidence Intervals (CI), and to assess the relative risk. A two-tailed P<0.05 was considered statistically significant.
Results
The demographic and clinicopathological features of IBD
We enrolled 210 patients in this study. The Receiver Operating Characteristic curve (ROC) analysis revealed that the optimal cut-off value of SIRI for active IBD was 0.86 × 109/L. The SIRI of 0.86 × 109/L had the highest sensitivity and specificity for active IBD. Hence, patients were stratified into 2 groups: a low SIRI group (SIRI<0.86 × 109/L) and a high SIRI group (SIRI ≥ 0.86 × 109/L). Table 1 shows the difference in demographic and clinicopathological features of the two SIRI groups. There are 107 IBD patients (51.0%) in the low SIRI group and 103 IBD patients (49.0%) in the high SIRI group. There were 119 males and 91 females, respectively. The median age was 31 y, range 27-51 y; a median BMI (Body Mass Index) was 20.1 Kg/m2, range 17.8-22.7 Kg/m2. A high SIRI was significantly correlated with active IBD (P=0.019), CRP (P=0.002), ESR (P<0.001), NLR (P<0.001) and MLR (P<0.001).
| Variables | low SIRI (n=107) | high SIRI (n=103) | χ2 | P |
|---|---|---|---|---|
| Age (y) | 1.913 | 0.167 | ||
| <31 | 49 (45.8%) | 57 (55.3%) | ||
| ≥ 31 | 58 (54.2%) | 46 (44.7%) | ||
| gender | 0.207 | 0.649 | ||
| Male | 59 (55.1%) | 60 (58.3%) | ||
| Female | 48 (44.9%) | 43 (41.7%) | ||
| BMI (Kg/m2) | 1.605 | 0.205 | ||
| <20.1 | 53 (49.5%) | 60 (58.3%) | ||
| ≥ 20.1 | 54 (50.5%) | 43 (41.7%) | ||
| IBD | 5.533 | 0.019 | ||
| Active | 46 (43.0%) | 61 (59.2%) | ||
| Inactive | 61 (57.0%) | 42 (40.8%) | ||
| UC (n=110) | 4.180 | 0.041 | ||
| Active | 24 (22.4%) | 28 (27.2%) | ||
| Inactive | 38 (35.5%) | 20 (19.4%) | ||
| CD (n=100) | 1.235 | 0.267 | ||
| Active | 22 (20.6%) | 33 (32.0%) | ||
| Inactive | 23 (21.5%) | 22 (21.4%) | ||
| Smoking | ||||
| Yes | 62 (57.9%) | 57 (55.3%) | 0.145 | 0.703 |
| No | 45 (42.1%) | 46 (44.7%) | ||
| Alcohol habit | 1.863 | 0.172 | ||
| Yes | 45 (42.1%) | 53 (51.5%) | ||
| No | 62 (57.9%) | 50 (48.5%) | ||
| CRP (mg/dl) | 9.239 | 0.002 | ||
| <33.5 | 64 (59.8%) | 40 (38.8%) | ||
| ≥ 33.5 | 43 (40.2%) | 63 (61.2%) | ||
| ESR (mm/H) | 13.891 | <0.001 | ||
| <23.5 | 67 (62.6%) | 38 (36.9%) | ||
| ≥ 23.5 | 40 (37.4%) | 65 (63.1%) | ||
| NLR | 14.866 | <0.001 | ||
| <2.20 | 72 (67.3%) | 42 (40.8%) | ||
| ≥ 2.20 | 35 (32.7%) | 61 (59.2%) | ||
| MLR | 11.950 | <0.001 | ||
| <0.25 | 65 (60.7%) | 38 (36.9%) | ||
| ≥ 0.25 | 42 (39.3%) | 65 (63.1%) |
Table 1. Demographic and clinicopathological features of two SIRI groups.
Disease severity of two SIRI groups
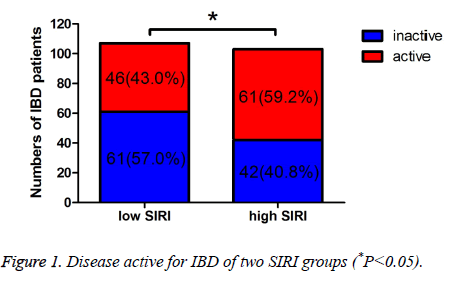
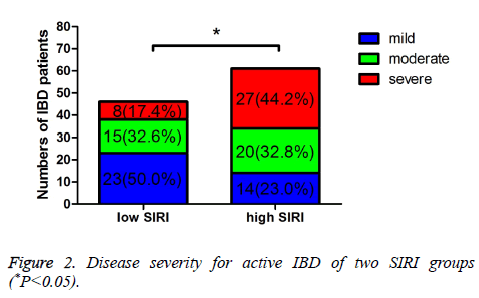
The Simple Clinical Colitis Activity Index (SCCAI) and CD Activity Index (CDAI) were used to evaluated the UC and CD activity, respectively. And the severity of UC and CD was evaluated by different scores of SCCAI or CDAI. In low SIRI group, 61 patients were inactive, 46 patients were active, respectively. In high SIRI group, 42 patients were inactive, 61 patients were active, respectively. Moreover, in low SIRI group, 23 patients were mild active, 15 patients were moderate active, 8 patients were severe active, respectively; in high SIRI group, 14 patients were mild active, 20 patients were moderate active, 27 patients were severe active, respectively. The active IBD was statistically significant between the two groups (P=0.019). Compared with the inactive IBD, the mild active and moderate active IBD were not statistically significant (P=0.907, 0.137), however, the severe active IBD was statistically significant (P<0.001) (Table 2 and Figure 1). Table 3 shows that the different severity of active IBD patients, and there was statistically significant of the different severity of active IBD between low SIRI group and high SIRI group (P=0.003) (Figure 2).
| Variables | Low SIRI (n=107) | High SIRI (n=103) | χ2 | P |
|---|---|---|---|---|
| Inactive | 61 (57.0%) | 42 (40.8%) | 5.533 | 0.019 |
| Mild | 23 (21.5%) | 14 (13.6%) | 0.014 | 0.907# |
| Moderate | 15 (14.0%) | 20 (19.4%) | 2.205 | 0.137# |
| Severe | 8 (7.5%) | 27 (26.2%) | 12.403 | <0.001# |
Table 2. Disease severity for IBD of two SIRI groups.
| Variables | Low SIRI (n=46) | High SIRI (n=61) | χ2 | P |
|---|---|---|---|---|
| Mild | 23 (50.0%) | 14 (23.0%) | 11.338 | 0.003 |
| Moderate | 15 (32.6%) | 20 (32.8%) | ||
| Severe | 8 (17.4%) | 27 (44.2%) |
Table 3. Disease severity for active IBD of two SIRI groups.
Univariate and multivariate analyses for IBD patients of SIRI
The univariate and multivariate logistic regression model analysis were used to explore the associations of the demographic and clinicopathological features with active IBD. Univariate analysis indicated that the CRP, ESR, SIRI, NLR and MLR had a statistically significant association with active IBD (Table 4). Multivariate analysis indicated that the CRP, ESR and SIRI were independently associated with active IBD (CRP: Hazard Ratios (HR), 1.874, 95% Confidence Intervals (CI), 1.545-2.438; ESR: HR, 1.612, 95% CI, 1.028-2.231; SIRI: HR, 1.865, 95% CI, 1.268-2.389; respectively) (Table 4). Therefore, the CRP, ESR and SIRI were the parameter capable of discriminating active from active IBD.
| Variables | Univariate analysis, HR (95% CI) | P | Multivariate analysis, HR (95% CI) | P |
|---|---|---|---|---|
| Age (y) | 0.786 | |||
| <31 | 1 (Reference) | |||
| ≥ 31 | 0.736 (0.542-1.204) | |||
| Gender | 0.378 | |||
| Male | 1 (Reference) | |||
| Female | 0.815 (0.671-1.158) | |||
| BMI (Kg/m2) | 0.705 | |||
| <20.1 | 1 (Reference) | |||
| ≥ 20.1 | 0.698 (0.520-1.218) | |||
| Smoking | 0.178 | |||
| Yes | 1 (Reference) | |||
| No | 0.925 (0.668-1.281) | |||
| Alcohol habit | 0.219 | |||
| Yes | 1 (Reference) | |||
| No | 1.278 (0.925-1.628) | |||
| CRP (mg/dl) | 0.004 | 0.022 | ||
| <33.5 | 1 (Reference) | 1 (reference) | ||
| ≥ 33.5 | 1.399 (1.005-1.795) | 1.874 (1.545-2.438) | ||
| ESR (mm/H) | 0.014 | 0.031 | ||
| <23.5 | 1 (Reference) | 1 (reference) | ||
| ≥ 23.5 | 1.545 (0.925-1.929) | 1.612 (1.028-2.231) | ||
| SIRI (× 109/L) | <0.001 | <0.001 | ||
| <0.86 | 1 (Reference) | 1 (reference) | ||
| ≥ 0.86 | 2.571 (1.935-3.124) | 1.865 (1.268-2.389) | ||
| NLR | <0.001 | 0.516 | ||
| <2.20 | 1 (Reference) | 1 (reference) | ||
| ≥ 2.20 | 2.055 (1.544-2.687) | 1.012 (0.798-1.727) | ||
| MLR | <0.001 | 0.729 | ||
| <0.25 | 1 (Reference) | 1 (reference) | ||
| ≥ 0.25 | 1.827 (1.109-2.318) | 0.866 (0.547-1.426) |
Table 4. Univariate and multivariate logistic regression model analyses for IBD of SIRI.
Discussion
Ulcerative colitis and Crohn’s disease, the main type of inflammatory bowel disease, are associated with dysregulated Inflammation and influence the gastrointestinal tract function [17]. There is a balance between the immune system and microbiota in the gastrointestinal tract, and the host immunity against or tolerate the innocuous antigens to keep the optimally balanced [18]. Histopathological examination plays a critical role in the diagnosis and management of IBD, however, this was expensive and invasive [19]. Thus, the endoscopic test, radiological test and laboratory are also used to further confirm the diagnose of IBD. Previous studies indicated that early detection of disease activity and severity could remarkably reduce the mortality of IBD [20-22]. IBD is also characterized by microbiome and environmental factors which are still poorly understood. Some researchers have suggested that the systemic inflammatory response may modulate the host microbiome of IBD, and has received increased attention [23,24]. Lots of studies have indicated that effective and efficient therapy could significantly control clinical symptoms (extraintestinal manifestations), reduce relapse, maintain remission, and improve the quality of life [25-27]. Therefore, selecting an ideal non-invasive test is increasingly expected and needed. As far as we are concerned, the SIRI value in patient with IBD has been rarely researched.
Accumulating evidence has demonstrated that the chemokines play a key role not only for homeostasis and optimally balance of immune system but also systemic inflammation [28]. Systemic inflammation is the result of chemokines, immune cells and inflammatory proteins. Chemokines are produced by many of cells including neutrophil, monocyte, lymphocyte and macrophage. These chemokines may present in IBD and play a remarkably role in enhancing the intestinal severity and systemic inflammation [29,30]. In the current study, we developed an SIRI based on the peripheral venous blood Neutrophil (N), Monocyte (M), and Lymphocyte (L) counts, and defined as N × M/L. The SIRI was used to predict the active and severity of IBD, and able to reflect the systemic inflammation. Systemic inflammation was that an increase of neutrophil, and accompanied by a relative decrease of lymphocyte. The neutrophil inhibits the immune system by the cytolytic activity of immune cells, and the lymphocyte improves the immune system via inducing cytotoxic cell death [31].
In the present study, we have demonstrated that the SIRI can predict the active and severity of patients with IBD, and we analysed the relationship between SIRI and demographic and clinicopathological features in patients with IBD. We observed that a high SIRI was significantly correlated with active IBD, CRP, ECR, NLR and MLR. The active IBD was statistically significant between low SIRI group and high SIRI group. Compared with the inactive IBD, the mild active and moderate active IBD were not statistically significant, however, the severe active IBD was statistically significant. Additionally, there was statistically significant of the different severity of active IBD between low SIRI group and high SIRI group. In univariate and multivariate logistic regression model analysis, we found that CRP, ESR and SIRI were independently associated with active IBD.
In this study, we used simple clinical colitis activity index and CD activity index to evaluate the UC and CD activity, respectively. These were widely used in clinical studies. To get accurately intestinal inflammation, we combined the endoscopic evaluation with tissue histology and laboratory markers to evaluate the active of IBD. Previous studies indicated that Neutrophil to Lymphocyte Ratio (NLR) was a useful biomarker of systemic inflammation responses for IBD [32]. The WBC, CRP and ESR were the most commonly inflammation factors to determine the IBD activity. And they could not adequately reflect disease activity since their low sensitivity and specificity for intestinal inflammation. However, there is no ideal marker for predicting the active and severity of IBD [33]. Recently, a new Systemic Inflammation Response Index (SIRI) based on neutrophil, monocyte, and lymphocyte counts was developed and acted as an independent predictor in many neoplastic diseases. And its prediction ability was shown to be better than NLR, MLR, LMR and other factors. Meanwhile, SIRI may provide early decisionmaking to the doctor to take effective therapy.
Recently, fecal lactoferrin, calprotectin and elastase were also used to evaluate the active IBD as novel inflammatory markers. Although the sensitivity and specificity in detecting the gastrointestinal inflammation, they are also not the ideal markers for IBD, and they are inconvenient to get the sample [34,35]. However, SIRI is a cost-effective and simple biomarker, and be an independent marker in this study, and reflects the active and severity of IBD. The neutrophils function of IBD may result in chronic inflammatory response, and associate with disease activity and epithelial injury of IBD. The lymphocyte function of IBD may influence by the neutrophil to be abnormal in intestinal mucosal [36].
This study has several limitations. Firstly, this study was a retrospective design and single-center design. Therefore, the results may not be representative of the general characteristic. Secondly, this study was enrolled a small number of patients. Hence, we should enrol a larger sample in further study. Thirdly, we have not compared the active IBD and clinical prognosis. Nevertheless, the peripheral blood results may provide a new sight to know the SIRI.
In conclusion, our study has demonstrated that the SIRI is evaluated in patients with IBD, and high SIRI may help the doctors to make decisions for patients with active IBD. SIRI is cost-effective and convenient indicator in IBD patients and may be a new promising marker of the disease severity in IBD. However, large multicenter studies are expected to assess changes in inflammatory markers in larger groups of patients with inflammatory bowel disease.
References
- Belkaid Y, Hand TW. Role of the microbiota in immunity and Inflammation. Cell 2014; 157: 121-141.
- Veronica M, Dylan K, Irving C. The Goldilocks Conundrum: NLR inflammasome modulation of gastrointestinal inflammation during inflammatory bowel disease. Crit Rev Immunol 2016; 36: 283-314.
- Davis BK, Philipson C, Hontecillas R, Eden K, Bassaganya-Riera J, Allen IC. Emerging significance of NLRs in inflammatory bowel disease. Inflamm Bowel Dis 2014; 20: 2412-2432.
- Kappelman MD, Farkas DK, Long MD, Erichsen R, Sandler RS, Sorensen HT, Baron JA. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol 2014; 12: 265-273.
- Rosenberg L, Lawlor GO, Zenlea T, Goldsmith JD, Gifford A, Falchuk KR, Wolf JL, Cheifetz AS, Robson SC, Moss AC. Predictors of endoscopic inflammation in patients with ulcerative colitis in clinical remission. Inflamm Bowel Dis 2013; 19: 779-784.
- Zhu J, Zhang F, Zhou J, Li H. Assessment of therapeutic response in Crohn's disease using quantitative dynamic contrast enhanced MRI (DCE-MRI) parameters: A preliminary study. Medicine (Baltimore) 2017; 96: 7759.
- Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflamm Bowel Dis 2013; 19: 789-799.
- Andersen NN, Jess T. Has the risk of colorectal cancer in inflammatory bowel disease decreased? World J Gastroenterol 2013; 19: 7561-7568.
- Ng WK, Wong SH, Ng SC. Changing epidemiological trends of inflammatory bowel disease in Asia. Intest Res 2016; 14: 111-119.
- Bruining DH, Loftus EV. Current and future diagnostic approaches: from serologies to imaging. Curr Gastroenterol Rep 2007; 9: 489-496.
- Feng JR, Qiu X, Wang F, Chen PF, Gao Q, Peng YN, Lin X, Liu Q, Liu J, Zhao Q, Li J. Diagnostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in Crohn's disease. Gastroenterol Res Pract 2017; 2017: 3526460.
- Sen BB, Rifaioglu EN, Ekiz O, Inan MU, Sen T, Sen N. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis. Cutan Ocul Toxicol 2013; 33: 223-237.
- Duan H, Zhang X, Wang FX, Cai MY, Ma GW, Yang H, Fu JH, Tan ZH, Meng YQ, Fu XY, Ma QL, Lin P. Prognostic role of neutrophil-lymphocyte ratio in operable esophageal squamous cell carcinoma. World J Gastroenterol 2015; 21: 5591-5597.
- Guclu H, Ozal SA, Pelitli Gurlu V, Birgul R. Elevated neutrophil lymphocyte ratio in recurrent optic neuritis. J Ophthalmol 2015; 2015: 758687.
- Baumgart DC, Metzke D, Schmitz J, Scheffold A, Sturm A, Wiedenmann B, Dignass AU. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut 2005; 54: 228-236.
- Best WR, Becktel JM, Singleton JW. Rederived values of the eight coefficients of the Crohn’s Disease Activity Index (CDAI). Gastroenterology 1979; 77: 843-846.
- Walsh AJ, Ghosh A, Brain AO, Buchel O, Burger D, Thomas S, White L, Collins GS, Keshav S, Travis SP. Comparing disease activity indices in ulcerative colitis. J Crohns Colitis 2014; 8: 318-325.
- Li Y, Nuij VJ, Baars JE, Biermann K, Kuipers EJ, Peppelenbosch MP, de Haar C, Janneke van der Woude C. Increased suppressor of cytokine signaling-3 expression predicts mucosal relapse in ulcerative colitis. Inflamm Bowel Dis 2013; 19: 132-140.
- Wong A, Bass D. Laboratory evaluation of inflammatory bowel disease. Curr Opin Pediatr 2008; 20: 566-570.
- Sandborn WJ, Loftus EV, Colombel JF, Fleming KA, Seibold F, Homburger HA, Sendid B, Chapman RW, Tremaine WJ, Kaul DK, Wallace J, Harmsen WS, Zinsmeister AR, Targan SR. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis 2001; 7: 192-201.
- Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 2008; 103: 162-169.
- Bruining DH, Loftus EV. Current and future diagnostic approaches: from serologies to imaging. Curr Gastroenterol Rep 2007; 9: 489-496.
- Xue TC, Zhang L, Xie XY, Ge NL, Li LX, Zhang BH, Ye SL, Ren ZG. Prognostic significance of the neutrophil-to-lymphocyte ratio in primary liver cancer: a meta-analysis. PLoS One 2014; 9: 96072.
- Sen BB, Rifaioglu EN, Ekiz O, Inan MU, Sen T, Sen N. Neutrophil to lymphocyte ratio as a measure of systemic inflammation in psoriasis. Cutan Ocul Toxicol 2013; 33: 223-237.
- Reinisch W, Sandborn WJ, Bala M, Yan S, Feagan BG, Rutgeerts P, Radford-Smith G, Xu S, Eisenberg D, Olson A, Colombel JF. Response and remission are associated with improved quality of life, employment and disability status, hours worked, and productivity of patients with ulcerative colitis. Inflamm Bowel Dis 2007; 13: 1135-1140.
- Card TR, Langan SM, Chu TP. Extra-gastrointestinal manifestations of inflammatory bowel disease may be less common than previously reported. Dig Dis Sci 2016; 61: 2619-2626.
- Koutroubakis IE. The relationship between coagulation state and inflammatory bowel disease: current understanding and clinical implications. Expert Rev Clin Immunol 2015; 11: 479-488.
- Kunkel EJ, Campbell DJ, Butcher EC. Chemokines in lymphocyte trafficking and intestinal immunity. Microcirculation 2003; 10: 313-323.
- Zimmerman NP, Vongsa RA, Wendt MK, Dwinell MB. Chemokines and chemokine receptors in mucosal homeostasis at the intestinal epithelial barrier in inflammatory bowel disease. Inflamm Bowel Dis 2008; 14: 1000-1011.
- Seegert D, Rosenstiel P, Pfahler H, Pfefferkorn P, Nikolaus S, Schreiber S. Increased expression of IL-16 in inflammatory bowel disease. Gut 2001; 48: 326-332.
- Milne K, Alexander C, Webb JR, Sun W, Dillon K, Kalloger SE, Gilks CB, Clarke B, Kobel M, Nelson BH. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med 2012; 10: 33.
- Torun S, Tunc BD, Suvak B, Yildiz H, Tas A, Sayilir A, Ozderin YO, Beyazit Y, Kayacetin E. Assessment of neutrophil-lymphocyte ratio in ulcerative colitis: a promising marker in predicting disease severity. Clin Res Hepatol Gastroenterol 2012; 36: 491-497.
- Khan K, Schwarzenberg SJ, Sharp H, Greenwood D, Weisdorf-Schindele S. Role of serology and routine laboratory tests in childhood inflammatory bowel disease. Inflamm Bowel Dis 2002; 8: 325-329.
- Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol 2008; 103: 162-169.
- Peterson CG, Sangfelt P, Wagner M, Hansson T, Lettesjo H, Carlson M. Fecal levels of leukocyte markers reflect disease activity in patients with ulcerative colitis. Scand J Clin Lab Invest 2007; 67: 810-820.
- Segal AW. How neutrophils kill microbes. Annu Rev Immunol 2005; 23: 197-223.