Research Article - Biomedical Research (2017) Volume 28, Issue 22
Synthesis of new pyridopyrimidinone-based thiadiazoles and pyrazolines as potential anti-breast cancer agents
Sobhi M. Gomha1*, Magda A. Abdallah1, Salim S. Al-Showiman2, Mahmoud A. Morad1 and Yahia N. Mabkhot2*
1Department of Chemistry, Faculty of Science, Cairo University, Giza, Egypt
2Department of Chemistry, College of Science, King Saud University, Riyadh-11451, Saudi Arabia
- *Corresponding Authors:
- Sobhi M. Gomha
Department of Chemistry
Cairo University, Egypt
Yahia N. Mabkhot
Department of Chemistry
College of Science
King Saud University, Saudi Arabia
Accepted date: October 16, 2017
Abstract
A series of novel thiadiazoles bearing pyrido [2,3-d] pyrimidinone moiety were synthesized by reaction of 2-(4-oxo-5, 7-di-p-tolyl-3, 4-dihydropyrido [2,3-d] pyrimidin-2-yl)-N-phenylhydrazine carbothioamide with a variety of hydrazonoyl chlorides in ethanol containing catalytic amount of triethylamine. Also, a novel pyrazolinone containing pyridopyrimidinone moiety was prepared by treating 2-hydrazinyl-5, 7- di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H) one with ethyl acetoacetate, and was utilized for synthesis of some new arylidene- and arylazo pyrazolinone derivatives. The structure of all the new products was established by elemental and spectroscopic analysis. The mechanisms of the studied reactions were also discussed. The antitumor activity of nine of the synthesized compounds was estimated versus MCF-7 cell line and the outcomes showed that some of them revealed powerful activity. In addition, the relation between the structure and the activity has been discussed.
Keywords
1, 3, 4-Thiadiazole, Pyrazolinone, Pyrido [2,3-d] pyrimidinone, Hydrazonoyl halides
Introduction
Pyridopyrimidine and its derivatives have a variety of chemical and biological significance as antimicroibial, analgesic, antiallergic, antitumor, antihypertensive, antileishmanial, antifolate, anti-inflammatory, ant-tuber-culostatic, anticonvulsant, diuretic, potassium sparing, and anti-aggressive activities [1-10]. 1, 3, 4-thiadiazole derivatives have attracted considerable interest owing to their wide spectra of biological activities such as antibacterial, antifungal, antituberculosis, antihepatitis B viral, antileishmanial, anti-inflammatory, analgesic, CNS depressant, anticancer, antioxidant, antidiabetic, molluscicidal, antihypertensive, diuretic, analgesic, antimicrobial, antitubercular, and anticonvulsant activities [11-20]. On the other hand, pyrazolines have been reported to possess a variety of significant and diverse pharmacological activities such as antibacterial, antifungal, anticonvulsant, antiviral, antitubercular, antidepressant, antiinflammatory, antiamoebic, analgesic and anticancer activities [21-30]. In view of these observations and in continuation of our previous work directed to the synthesis of novel heterocyclic compounds of potential biological and pharmacological activities, we reported here facile route for the synthesis of some new thiadiazole and pyrazoline derivatives containing pyridopyrimidine moiety.
Materials and Methods
Chemistry
Measurements of the melting points were carried out on Electrothermal IA 9000 series digital melting point apparatus. The IR spectra were recorded in potassium bromide discs on a Pye Unicam SP 3300 and Shimadzu FT-IR 8101 PC infrared spectrophotometer. 1H-NMR and 13C-NMR spectra were measured in deuterated dimethyl sulfoxide (DMSO-d6) using a Varian Gemini 300 NMR spectrometer. Mass spectra were recorded on a Shimadzu GCMS-QP1000 EX mass spectrometer at 70 eV. Measurements of the elemental analysis were carried out at the Microanalytical Centre of Cairo University, Giza, Egypt. All reactions were followed by TLC (Silica gel, Merck). The biological evaluation of the products was carried out at the Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt.
Synthesis of 2-(4-oxo-5, 7-di-p-tolyl-3, 4-dihydropyrido[2,3- d] pyrimidin-2-yl)-N-phenyl hydrazinecarbothioamide (3): A mixture of 2-hydrazinyl-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (3.57 g, 10 mmol) and phenyl isothiocyanate 2 (1.35 g, 10 mmol) in absolute ethanol (50 ml) was refluxed for 4 h. The formed solid after cooling was filtered off, washed with ethanol, dried and finally crystallized from ethanol to give pure product of compound 3, as white crystals (70%); m.p. 202-204°C; IR (KBr): v 3322, 3214, 3125 (4 NH), 3023, 2918 (CH), 1651 (C=O), 1592 (C=N) cm-1; 1HNMR (DMSO-d6): δ 2.22 (s, 3H, CH3), 2.36 (s, 3H, CH3), 7.06-7.95 (m, 15 H, Ar-H, pyridine H-5 and 1 NH), 8.53 (s, br, 1H, NH, D2O-exchangeable), 9.42 (s, br, 1H, NH, D2Oexchangeable), 10.09 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 492 (M+, 15), 383 (19), 307 (38), 117 (49), 59 (100). Anal Calcd for C28H24N6OS (492.17): C, 68.27; H, 4.91; N, 17.06. Found: C, 68.03; H, 4.86; N, 17.01%.
Synthesis of 1, 3, 4-thiadiazole derivatives (7a-j): A mixture of compound 3 (0.492 g, 1 mmol) and the appropriate hydrazonoyl halides 4a-4j (1 mmol) in ethanol (20 ml) containing triethylamine (0.1 g, 1 mmol) was refluxed for 4-6 h. (monitored by TLC). The formed precipitate was isolated by filtration, washed with methanol, dried and recrystallized from DMF to give products 7a-7j. The physical constants and spectral data of the obtained products are listed below:
2-(2-(5-Acetyl-3-phenyl-1, 3, 4-thiadiazol-2 (3H)-ylidene) hydrazinyl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)- one (7a): Brown solid (76%); m.p. 124-126°C; IR (KBr): v 3414, 3160 (2 NH), 3027, 2959 (CH), 1712, 1651 (2 C=O), 1598 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.22 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.46 (s, 3H, CH3), 7.10-7.88 (m, 14 H, Ar-H and pyridine-H5), 9.47 (s, br, 1H, NH, D2O-exchangeable), 11.18 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 559 (M+, 26), 354 (97), 311 (50), 247 (68), 117 (51), 92 (100), 59 (93). Anal. Calcd. for C31H25N7O2S (559.18): C, 66.53; H, 4.50; N, 17.52. Found C, 66.56; H, 4.39; N, 17.42%.
2-(2-(5-Acetyl-3-(p-tolyl)-1, 3, 4-thiadiazol-2 (3H)-ylidene) hydrazinyl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)- one (7b): Brown solid (73%); m.p. 138-140°C; IR (KBr): v 3403, 3157 (2 NH), 3021, 2919 (CH), 1710, 1648 (2 C=O), 1596 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.17 (s, 3H, CH3), 2.37 (s, 3H, CH3), 2.42 (s, 3H, CH3), 2.47 (s, 3H, CH3), 7.08-7.91 (m, 13H, Ar-H and pyridine-H5), 9.38 (s, br, 1H, NH, D2O-exchangeable), 11.22 (s, br, 1H, NH, D2Oexchangeable); MS m/z (%): 573 (M+, 18), 380 (64), 261 (50), 106 (100), 91 (80), 59 (66). Anal. Calcd. for C32H27N7O2S (573.19): C, 67.00; H, 4.74; N, 17.09. Found C, 66.95; H, 4.71; N, 16.93%.
2-(2-(5-Acetyl-3-(4-chlorophenyl)-1, 3, 4-thiadiazol-2 (3H)- ylidene) hydrazinyl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (7c): Brown solid (77%); m.p. 147-149°C; IR (KBr): v 3412, 3322 (2 NH), 3028, 2945 (CH), 1719, 1673 (2 C=O), 1596 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.26 (s, 3H, CH3), 2.36 (s, 3H, CH3), 2.50 (s, 3H, CH3), 7.06-7.89 (m, 13H, Ar-H and pyridine-H5), 10.09 (s, br, 1H, NH, D2O-exchangeable), 11.19 (s, br, 1H, NH, D2Oexchangeable); MS m/z (%): 595 (M+ +2, 6), 593 (M+, 20), 486 (62), 250 (46), 118 (74), 91 (100), 77 (84). Anal. Calcd. for C31H24ClN7O2S (593.14): C, 62.67; H, 4.07; N, 16.50. Found C, 62.49; H, 4.02; N, 16.36%.
2-(2-(5-Acetyl-3-(3-chlorophenyl)-1, 3, 4-thiadiazol-2 (3H)- ylidene) hydrazinyl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (7d): Brown solid (70%); m.p. 122-124°C; IR (KBr): v 3388, 3247 (2 NH), 3024, 2919 (CH), 1710, 1677 (2 C=O), 1595 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.23 (s, 3H, CH3), 2.36 (s, 3H, CH3), 2.47 (s, 3H, CH3), 6.63-7.89 (m, 13H, Ar-H and pyridine-H5), 10.09 (s, br, 1H, NH, D2O-exchangeable), 11.11 (s, br, 1H, NH, D2Oexchangeable); MS m/z (%): 595 (M+ +2, 9), 593 (M+, 31), 477 (60), 250 (51), 118 (46), 91 (100), 77 (47). Anal Calcd. for C31H24ClN7O2S (593.14): C, 62.67; H, 4.07; N, 16.50. Found C, 62.45; H, 4.12; N, 16.37%.
2-(2-(5-Acetyl-3-(4-bromophenyl)-1, 3, 4-thiadiazol-2 (3H)- ylidene) hydrazinyl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (7e): Brown solid (74%); m.p. 144-146°C; IR (KBr): v 3416, 3212 (2 NH), 3022, 2919 (CH), 1713, 1676 (2 C=O), 1595 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.25 (s, 3H, CH3), 2.35 (s, 3H, CH3), 2.47 (s, 3H, CH3), 6.90-8.06 (m, 13H, Ar-H and pyridine-H5), 10.08 (s, br, 1H, NH, D2O-exchangeable), 11.13 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 639 (M+ +2, 12), 637 (M+, 16), 458 (64), 248 (33), 118 (69), 91 (100), 77 (73). Anal. Calcd. for C31H24BrN7O2S (637.09): C, 58.31; H, 3.79; N, 15.35. Found C, 58.24; H, 3.70; N, 15.26%.
Ethyl 5-(2-(4-oxo-5, 7-di-p-tolyl-3, 4-dihydropyrido [2,3-d] pyrimidin-2-yl) hydrazono)-4-phenyl-4, 5-dihydro-1, 3, 4- thiadiazole-2-carboxylate (7f): Yellow solid (70%); m.p. 120-122°C; IR (KBr): v 3413, 3196 (2 NH), 3027, 2921 (CH), 1702, 1678 (2 C=O), 1598 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 1.30 (t, 3H, J=7.14 Hz, CH2CH3), 2.21 (s, 3H, CH3), 2.36 (s, 3H, CH3), 4.19 (q, 2 H, J=7.14 Hz, CH2CH3), 6.97-7.89 (m, 14 H, Ar-H and pyridine-H5), 10.09 (s, br, 1H, NH, D2O-exchangeable), 11.04 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 589 (M+, 20), 405 (17), 293 (19), 176 (31), 87 (84), 59 (100). Anal. Calcd. for C32H27N7O3S (589.19): C, 65.18; H, 4.62; N, 16.63. Found C, 65.04; H, 4.61; N, 16.55%.
Ethyl 4-(4-chlorophenyl)-5-(2-(4-oxo-5, 7-di-p-tolyl-3, 4- dihydropyrido (2,3-d]pyrimidin-2-yl) hydrazono)-4, 5- dihydro-1, 3, 4-thiadiazole-2-carboxylate (7g): Yellow solid (73%); m.p. 162-164°C; IR (KBr): v 3428, 3199 (2 NH), 3027, 2921 (CH), 1738, 1670 (2 C=O), 1596 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 1.18 (t, 3H, J=7.14 Hz, CH2CH3), 2.26 (s, 3H, CH3), 2.32 (s, 3H, CH3), 4.22 (q, 2H, J=7.14 Hz, CH2CH3), 7.06-7.86 (m, 13H, Ar-H and pyridine-H5), 10.07 (s, br, 1H, NH, D2O-exchangeable), 11.26 (s, br, 1H, NH, D2Oexchangeable); MS m/z (%): 625 (M+ +2, 7), 623 (M+, 24), 357 (19), 516 (63), 248 (38), 118 (53), 91 (75), 64 (100). Anal. Calcd. for C32H26ClN7O3S (623.15): C, 61.58; H, 4.20; N, 15.71. Found C, 61.51; H, 4.13; N, 15.63%.
Ethyl 4-(2,4-dichlorophenyl)-5-(2-(4-oxo-5, 7-di-p-tolyl-3, 4- dihydropyrido (2,3-d]pyrimidin-2-yl) hydrazono)-4, 5- dihydro-1, 3, 4-thiadiazole-2-carboxylate (7h): Yellow solid (77%); m.p. 151-153°C; IR (KBr): v 3413, 3197 (2 NH), 3027, 2920 (CH), 1730, 1677 (2 C=O), 1590 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 1.28 (t, 3H, J=7.14 Hz, CH2CH3), 2.26 (s, 3H, CH3), 2.36 (s, 3H, CH3), 4.27 (q, 2 H, J=7.14 Hz, CH2CH3), 6.99-7.89 (m, 12 H, Ar-H and pyridine-H5), 10.09 (s, br, 1H, NH, D2O-exchangeable), 11.20 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 657 (M+, 9), 545 (31), 352 (63), 250 (52), 117 (54), 87 (100), 59 (68). Anal. Calcd. for C32H25Cl2N7O3S (657.11): C, 58.36; H, 3.83; N, 14.89. Found C, 58.27; H, 3.74; N, 14.81%.
5-(2-(4-Oxo-5, 7-di-p-tolyl-3, 4-dihydropyrido [2,3-d] pyrimidin-2-yl) hydrazono)-N, 4-diphenyl-4, 5-dihydro-1, 3, 4-thiadiazole-2-carboxamide (7i): Yellow solid (73%); m.p. 151-153°C; IR (KBr): v 3405, 3320, 3186 (3 NH), 3029, 2915 (CH), 1683, 1649 (2 C=O), 1594 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.26 (s, 3H, CH3), 2.36 (s, 3H, CH3), 7.06-7.89 (m, 19H, Ar-H and pyridine-H5), 10.09 (s, br, 1H, NH, D2Oexchangeable), 10.74 (s, br, 1H, NH, D2O-exchangeable), 11.15 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 636 (M+, 13), 352 (62), 250 (43), 128 (100), 91 (93), 77 (64). Anal. Calcd. for C36H28N8O2S (636.21): C, 67.91; H, 4.43; N, 17.60. Found C, 67.73; H, 4.41; N, 17.45%.
2-(2-(3, 5-Diphenyl-1, 3, 4-thiadiazol-2 (3H)-ylidene) hydrazinyl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)- one (7j): Yellow solid (70%); m.p. 194-196°C; IR (KBr): v 3409, 3212 (2 NH), 3029, 2917 (CH), 1653 (C=O), 1594 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.27 (s, 3H, CH3), 2.36 (s, 3H, CH3), 7.02-7.83 (m, 19 H, Ar-H and pyridine-H5), 10.06 (s, br, 1H, NH, D2O-exchangeable), 10.72 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 593 (M+, 19), 420 (47), 313 (58), 226 (91), 105 (72), 77 (100). Anal. Calcd. for C35H27N7OS (593.20): C, 70.81; H, 4.58; N, 16.51. Found C, 70.77; H, 4.51; N, 16.38%.
Alternate synthesis of 7j: A mixture of equimolar amounts of 2-(methylthio)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)- one (9) (0.373 g, 1 mmol) and 2-hydrazono-3, 5-diphenyl-2,3- dihydro-1, 3, 4-thiadiazole (10) (0.268 g, 1 mmol) in ethanol (20 ml) was refluxed till all of the starting materials have been disappeared and methanethiol ceased to evolve (12 h, monitored by TLC). The solvent was evaporated and the residue was triturated with methanol. The solid that formed was filtered and recrystallized from DMF to give compound 7j that was identical in all respects (m.p., mixed m.p. and IR spectra) with that obtained from reaction of 3 with 4j.
Synthesis of 2-(3-methyl-5-oxo-4, 5-dihydro-1H-pyrazol-1- yl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (11): A mixture of 2-hydrazinyl-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (1) (3.57 g, 10 mmol) and ethyl acetoacetate (1.22 g, 10 mmol) in acetic acid (30 ml) was refluxed for 6 h. The product started to separate out during the course of the reaction. The solid product was filtered, washed with water, dried and recrystallized from EtOH to give the corresponding pyrazoline derivative 11 as yellow solid (80%); m.p. 144-146°C; IR (KBr): v 3431 (NH), 3021, 2927 (CH), 1776, 1658 (2 C=O), 1595 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.24 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.36 (s, 3H, CH3), 3.83 (s, 2 H, CH2), 7.01-7.79 (m, 9 H, Ar-H and pyridine-H5), 10.09 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 423 (M+, 13), 248 (66), 119 (100), 91 (72), 77 (39). Anal. Calcd. for C25H21N5O2 (423.17): C, 70.91; H, 5.00; N, 16.54. Found C, 70.69; H, 4.88; N, 16.38%.
Coupling of 11 with arenediazonium chlorides 12a-12d: A stirred solution of pyrazolinone 11 (0.423 g, 1 mmol) in pyridine (10 ml) was cooled in an ice bath to 0-5°C. To the resulting solution, while being stirred, was added dropwise over a period of 10 min a solution of the appropriate arenediazonium chloride, prepared as usual by diazotizing the respective arylamine (1 mmol) in hydrochloric acid (6 M, 1 ml) with sodium nitrite (1 M, 1 ml). The whole mixture was then left in a refrigerator overnight. The precipitated solid was collected, washed with water and finally crystallized from ethanol to give the respective hydrazone 13a-13d.
2- (3-Methyl-5-oxo-4- (2-phenylhydrazono)-4, 5- dihydro-1H-pyrazol-1-yl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (13a) : Yellow solid (70%); m.p. 150-152°C; IR (KBr): v 3225, 3252 (2 NH), 3023, 2918 (CH), 1661, 1643 (2 C=O), 1604 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.24 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.36 (s, 3H, CH3), 6.87-7.91 (m, 14 H, Ar-H and pyridine-H5), 10.07 (s, br, 1H, NH, D2O-exchangeable), 10.63 (s, br, 1H, NH, D2Oexchangeable); MS m/z (%): 527 (M+, 23), 458 (50), 341 (74), 215 (61), 158 (39), 117 (100). Anal. Calcd. for C31H25N7O2 (527.21): C, 70.57; H, 4.78; N, 18.58. Found C, 70.51; H, 4.69; N, 18.38%.
2-(3-methyl-5-oxo-4-(2-(p-tolyl) hydrazono)-4, 5- dihydro-1H-pyrazol-1-yl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (13b): Yellow solid (73%); m.p. 139-141°C; IR (KBr): v 3426, 3237 (2 NH), 3023, 2922 (CH), 1671, 1638 (2 C=O), 1604 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.25 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.33 (s, 3H, CH3), 2.36 (s, 3H, CH3), 7.06-7.88 (m, 13H, Ar-H and pyridine-H5), 10.07 (s, br, 1H, NH, D2O-exchangeable), 10.63 (s, br, 1H, NH, D2Oexchangeable); MS m/z (%): 541 (M+, 23), 458 (79), 368 (91), 240 (45), 117 (100), 78 (60). Anal. Calcd. for C32H27N7O2 (541.22): C, 70.96; H, 5.02; N, 18.10. Found C, 70.82; H, 5.01; N, 17.93%.
2-(4-(2-(4-methoxyphenyl) hydrazono)-3-methyl-5-oxo-4, 5- dihydro-1H-pyrazol-1-yl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (13c): Yellow solid (71%); m.p. 130-132°C; IR (KBr): v 3383, 3226 (2 NH), 3022, 2918 (CH), 1662, 1638 (2 C=O), 1604 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.25 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.36 (s, 3H, CH3), 3.85 (s, 3H, OCH3), 7.02-7.89 (m, 13H, Ar-H and pyridine-H5), 10.06 (s, br, 1H, NH, D2O-exchangeable), 10.57 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 557 (M+, 20), 458 (74), 368 (100), 229 (59), 140 (36), 82 (40). Anal. Calcd. for C32H27N7O3 (557.22): C, 68.93; H, 4.88; N, 17.58. Found C, 68.90; H, 4.75; N, 17.47%.
2-(4-(2-(4-chlorophenyl) hydrazono)-3-methyl-5-oxo-4, 5- dihydro-1H-pyrazol-1-yl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (13d): Yellow solid (74%); m.p. 166-168°C; IR (KBr): v 3379, 3223 (2 NH), 3023, 2918 (CH), 1662, 1655 (2 C=O), 1604 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.24 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.36 (s, 3H, CH3), 6.87-7.92 (m, 13H, Ar-H and pyridine-H5), 10.09 (s, br, 1H, NH, D2O-exchangeable), 10.62 (s, br, 1H, NH, D2Oexchangeable); MS m/z (%): 563 (M+ +2, 6), 561 (M+, 21), 453 (60), 351(53), 249 (43), 163 (100), 105 (28), 59 (67). Anal. Calcd. for C31H24ClN7O2 (561.17): C, 66.25; H, 4.30; N, 17.45. Found C, 66.13; H, 4.20; N, 17.39%.
Reaction of 11 with arylaldehydes 14a-14d: A mixture of pyrazolinone 11 (0.423 g, 1 mmol) and the appropriate aldehyde 14a-14d (1 mmol) in pyridine (10 ml) was refluxed for 4-8 h (monitored by TLC). The solvent was collected by distillation and the residue was treated with ice/HCl mixture. The solid product was collected, washed with ethanol, dried, and finally recrystallized from ethanol to give the respective compound 15a-15d. The products 15a-15d together with their physical constants are listed below:
2-(4-Benzylidene-3-methyl-5-oxo-4, 5-dihydro-1Hpyrazol- 1-yl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)- one (15a): Yellow solid (68%); m.p. 161-163°C; IR (KBr): v 3421 (NH), 3023, 2919 (CH), 1661, 1641 (2 C=O), 1604 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.25 (s, 3H, CH3), 2.28 (s, 3H, CH3), 2.36 (s, 3H, CH3), 7.06-7.97 (m, 14H, Ar-H and pyridine-H5), 8.18 (s, 1H, CH=), 10.07 (s, br, 1H, NH, D2Oexchangeable); MS m/z (%): 511 (M+, 13), 264 (49), 203 (85), 103 (51), 44 (100). Anal. Calcd. for C32H25N5O2 (511.20): C, 75.13; H, 4.93; N, 13.69. Found C, 75.10; H, 4.83; N, 13.61%.
2-(3-Methyl-4-(4-methylbenzylidene)-5-oxo-4, 5- dihydro-1H-pyrazol-1-yl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (15b): Yellow solid (69%); m.p. 147-149°C; IR (KBr): v 3426 (NH), 3023, 2921 (CH), 1683, 1638 (2C=O), 1604 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.24 (s, 3H, CH3), 2.29 (s, 3H, CH3), 2.33 (s, 3H, CH3), 2.36 (s, 3H, CH3), 6.86-7.88 (m, 13H, Ar-H and pyridine-H5), 8.15 (s, 1H, CH=), 10.04 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 525 (M+, 27), 355 (100), 234 (48), 172 (38), 94 (59). Anal. Calcd. for C33H27N5O2 (525.22): C, 75.41; H, 5.18; N, 13.32. Found C, 75.27; H, 5.10; N, 13.27%.
2-(4-(4-Methoxybenzylidene)-3-methyl-5-oxo-4, 5- dihydro-1H-pyrazol-1-yl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (15c): Yellow solid (67%); m.p. 138-140°C; IR (KBr): v 3428 (NH), 3023, 2921 (CH), 1673, 1629 (2 C=O), 1604 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.24 (s, 3H, CH3), 2.33 (s, 3H, CH3), 2.36 (s, 3H, CH3), 3.86 (s, 3H, OCH3), 6.94-7.88 (m, 13H, Ar-H and pyridine-H5), 8.14 (s, 1H, CH=), 9.87 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 541 (M+, 15), 313 (47), 236 (43), 119 (100), 83 (59). Anal. Calcd. for C33H27N5O3 (541.21): C, 73.18; H, 5.02; N, 12.93. Found C, 73.09; H, 5.07; N, 12.75%.
2-(4-(4-Chlorobenzylidene)-3-methyl-5-oxo-4, 5- dihydro-1H-pyrazol-1-yl)-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H)-one (15d): Yellow solid (69%); m.p. 173-175°C; IR (KBr): v 3416 (NH), 3023, 2918 (CH), 1665, 1642 (2 C=O), 1602 (C=N) cm-1; 1H-NMR (DMSO-d6): δ 2.24 (s, 3H, CH3), 2.33 (s, 3H, CH3), 2.38 (s, 3H, CH3), 6.86-7.92 (m, 13H, Ar-H and pyridine-H5), 8.20 (s, 1H, CH=), 10.06 (s, br, 1H, NH, D2O-exchangeable); MS m/z (%): 547 (M+ +2, 6), 545 (M+, 15), 458 (37), 368 (49), 262 (61), 69 (100), 57 (94). Anal. Calcd. for C32H24ClN5O2 (545.16): C, 70.39; H, 4.43; N, 12.83. Found C, 70.31; H, 4.29; N, 12.68%.
Anticancer activity
The cytotoxic evaluation of the synthesized compounds was carried out at the Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt according to the reported method [31].
Results and Discussion
Chemistry
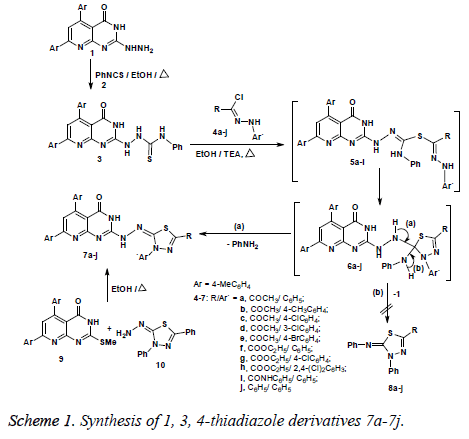
2-Hydrazinyl-5, 7-di (p-tolyl) pyrido [2,3-d] pyrimidin-4 (3H) one (1) was prepared as previously described, by reaction of 2- thioxo -5, 7-di (p-tolyl) pyrido [2,3-d] pyrimidin-4 (3H)-one with hydrazine hydrate under reflux in ethanol (Scheme 1) [32]. Treatment of 2-hydrazinyl-5, 7-di(p-tolyl) pyrido [2,3-d] pyrimidin-4 (3H) one (1) with phenyl isothiocyanate (2) under reflux in ethanol for 4 h afforded product 3, namely, 2-(4- oxo-5, 7-di-p-tolyl-3, 4-dihydropyrido [2,3-d] pyrimidin-2-yl)- N-phenylhydrazine carbothioamide (Scheme 1). The structure assigned for compound 3 was established based on elemental analysis and spectral data. For example, the IR spectrum of 3 revealed the presence of three absorption bands at vmax 3322, 3214, 3125 cm-1 assigned for the 4 NH groups, in addition to another absorption band at vmax 1651 cm-1 due to the carbonyl group of the pyrimidinone ring. 1H NMR spectrum of 3 exhibited four signlet signals at δ 7.95, 8.53, 9.42 and 10.09 ppm attributed to the 4NH protons, in addition to two signals at δ 2.22 and 2.36 ppm assigned for the two methyl groups. Mass spectrum of 3 revealed a molecular ion peak at m/z=492 which is in agreement with the proposed structure. The presence of the thioamidehydrazine moiety as a side chain in compound 3 prompted us to utilize it for constructing 1, 3, 4-thiadiazole ring via its reaction with hydrazonoyl halides [33]. Thus, reaction of compound 3 with the appropriate hydrazonoyl chlorides 4a-4j under reflux in ethanol in the presence of triethylamine as a basic catalyst led to formation of products 7a-7j, and not the other products 8 (Scheme 1). The elemental analysis together with the data derived from IR, 1H NMR and mass spectra are in agreement with the proposed structure 7. The IR spectra of products 7 showed in each case the presence of two absorption bands near vmax 1712, 1651 cm-1 for the two carbonyl groups, in addition to another two bands near vmax 3414, 3160 cm-1 for the 2 NH groups.
The 1H NMR spectra of 7 revealed the presence of two broad singlet signals near δ 9.47, 11.18 ppm, assigned for the 2 NH protons, in addition to the expected signals for the protons of the two methyl groups, the acetyl group (or ester group) at position -2 of the 1, 3, 4-thiadiazole ring and the aryl protons. The mass spectrum of each of products 7 revealed the presence of a molecular ion peak (m/z) which is consistent with the structure of the respective compound (see experimental section). The structure assigned for products 7 was further evidenced via alternative method. Thus, reaction of 2- methylthio-5, 7-di-p-tolylpyrido [2,3-d] pyrimdin-4 (3H)-one 9 with 3, 5-diphenyl-1, 3, 4-thiadiazolylidene-2-hydrazine 10 in ethanol under reflux, afforded a product which is typical in all respects with that obtained from reaction of 3 with 4j (Scheme 1). To account for the formation of the product 7, it was suggested that the reaction of compound 3 with hydrazonoyl halide 4 initially gives the intermediate 5 by 1, 3-nucleophilic addition, followed by in situ cyclization to give 6, which in turn loses one molecule of aniline (route a) to give the final product 7. The other route (b) outlined in Scheme 1 is excluded since it leads to formation of product 8 via elimination of 1 from intermediate 6, which is completely different in all respects (IR, 1H NMR, mass spectra) from product 7.
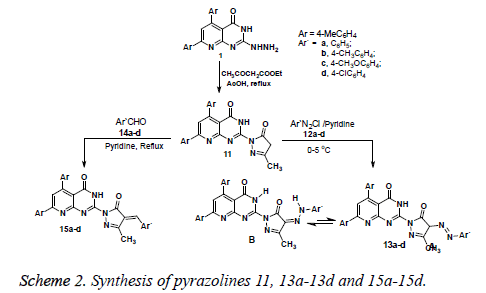
The starting compound 1 was then used for preparation of pyrazoline derivatives bearing pyridopyrimidine moiety. Thus, reaction of 2-hydrazinyl-5, 7-di-p-tolylpyrido [2,3-d] pyrimidin-4 (3H) one 1 with ethyl acetoacetate under reflux in acetic acid for 6 h led to formation of one isolable product as evidenced by TLC analysis of the crude product (Scheme 2). The structure of the product was identified to be 11 based on elemental analysis and spectral data. For example, IR spectrum of 11 revealed two absorption bands at vmax 1776, 1658 cm-1 due to the two carbonyl groups, and another absorption band at vmax 3431 cm-1 attributed to the -NH group. 1H NMR spectrum of 11 displayed singlet signal at δ 3.83, and another broad signal at δ 10.09 ppm assigned for the -CH2- and -NH protons, in addition to the expected signals assigned for the 2 CH3 and aryl protons. The mass spectrum of compound 11 revealed a molecular ion peak at m/z=423 which is in agreement with the proposed structure.
The presence of an active methylene group in the pyrazolinone ring (at position 4) in structure 11 can be used as an active site for electrophilic reactions. Thus, reaction of product 11 with a number of arenediazonium salts 12a-12d in pyridine at low temperature (0-5°C) afforded the respective arylazocompounds 13a-13d. The latter compounds were found to be existed mainly in the hydrazone-form (B) rather than the azoform (A) based on spectral data (IR and 1H NMR) (see Experimental section).
The reaction of pyrazolinone derivative 11 with a variety of substituted benzaldehyde 14a-14d under reflux in pyridine for 4-8 h afforded the corresponding arylidene compounds 15 (Scheme 2). The structure 15 assigned for the arylidene products was substantiated by elemental analysis and spectral data. For example, the IR spectra of compound 15 revealed in each case the presence of two absorption bands near vmax 1661, 1641 cm-1 due to the two carbonyl groups and one absorption band near vmax 3421 cm-1 assigned for the -NH group. The 1H NMR spectra of products 15 revealed the presence of a characteristic singlet signal at δ 8.18 ppm, assigned for the olefinic proton, in addition to the expected signals of the methyl and aryl protons. The mass spectra revealed a molecular ion peak at the correct value for each arylidene derivative.
Biological activity
The cytotoxic evaluation of the synthesized compounds was carried out at the Regional Center for Mycology and Biotechnology at Al-Azhar University, Cairo, Egypt according to the reported method [33]. Cytotoxicity versus human breast cancer MCF-7 cell line. The antiproliferative activity of the target thiazoles was screened against human breast MCF-7 cancer cell line using Sulforhodamine-B (SRB) assay, and the reference drug is tamoxifen. Data generated were used to plot a dose response curve of which the concentration of test compounds required to kill 50% of cell population (IC50) was determined. The results depicted in Table 1 revealed that all the tested compounds showed inhibitory activity against the breast carcinoma cell line (MCF7) in a concentration dependent manner. Cytotoxic activity was expressed as the mean IC50 of three independent experiments.
| Tested compounds | IC50 values (μg/ml) | Tested compounds | IC50 values (μg/ml) |
|---|---|---|---|
| 7a | 14.9 ± 1.93 | 7j | 63.1 ± 3.62 |
| 7b | 9.7 ± 1.19 | 13a | 9.4 ± 2.3 |
| 7c | 20.6 ± 3.8 | 13b | 8.1 ± 1.7 |
| 7f | 8.7 ± 1.28 | 15a | 11.1 ± 1.6 |
| 7i | 10.5 ± 1.73 | Tamoxifen | 8.1 ± 1.23 |
Table 1. Effect of the synthesized compounds on MCF-7 cell line.
Examination of the compound activities leads to the following conclusions
• The activities of the synthesized compounds depend on the structural skeleton and electronic environment of the molecules.
• Based on our limited study, the pyrazolinone ring as in 13 and 15 has in vitro inhibitory activity greater than the 1, 3, 4-thiadiazole ring in 7.
For 1,3,4-Thiadiazoles 7a–j
• The in vitro inhibitory activity of compounds with substituents at position 5 is in the order of: COOEt>CONHPh>CH3CO>Ph (7f>7a>7i >7j).
• The introduction of electron-donating group (methyl) at C4 of the phenyl group at position 4 in the 1, 3, 4-thiadiazole ring enhances the antitumor activity. In contrast, introduction of an electron-withdrawing group (chlorine) decreases the antitumor activity (7b>7a>7c).
For pyrazolinones 13a-13d and 15a-15d
• For substituent at position 4: the arylhydrazo group (ArNHN=) gives higher activity than the arylidene group (Ar-CH=) (13>15).
For arylhydrazopyrazolinones: the electron-donating group (methyl) at C4 of the phenyl ring enhances the antitumor activity (13b>13a).
Conclusion
In this context, a series of novel thiadiazoles and pyrazolinones bearing pyridopyrimidine moiety were synthesized by adopting simple methods and by using 2-hydrazinyl-5, 7-di-ptolylpyrido [2,3-d] pyrimidinone as the starting compound. The structure of all the newly prepared products was established based on both elemental analysis and spectral data and by alternative method wherever possible. Moreover, nine synthesized compounds were estimated for their antitumor activity versus breast cancer cell line. Four derivatives were found to have moderate to high anticancer activity. Although the mechanism of action of these compounds as anticancer agents wasn't investigated here due to financial obstacles, this of course will be given a full consideration in a future related works as possible as we can.
Acknowledgments
This project was supported by King Saud University, Deanship of Scientific Research, College of Science research center.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- Cordeu L, Cubedo E, Bandres E, Rebollo A, Saenz X, Chozas H, Dominguez MV, Echeverria M, Mendivil B, Sanmartin C, Palop JA, Font M, Foncillas JG. Biological profile of new apoptotic agents based on 2, 4-pyrido [2,3-d] pyrimidine derivatives. J Bioorg Med Chem 2007; 15, 1659-1669.
- Font M, Gonzalez A, Palop JA, Sanmartin C. New insights into the structural requirements for pro-apoptotic agents based on 2, 4-diaminoquinazoline, 2, 4-diaminopyrido [2,3-d] pyrimidine and 2, 4-diaminopyrimidine derivatives. Eur J Med Chem 2011; 46: 3887-3899.
- Dorsey JF, Jove R, Kraker AJ, Wu J. The pyrido [2,3-d] pyrimidine derivative PD180970 inhibits p210Bcr-Abl tyrosine kinase and induces apoptosis of K562 leukemic cells. J Cancer Res 2000; 60: 3127-3131.
- Deyanov AB, Niyazov RK, Nazmetdivov FY, Syropyatov BY, Kolla VE, Konshin ME. Synthesis and biological activity of amides and nitriles of 2- arylami-no-5-carboxy(carbethoxy)-6-methylnicotinic acids and 1-aryl-6-carbethoxy-7-methyl-4-oxo-1, 4-dihydro- pyri-do [2,3-d] pyrimidines. J Pharm Chem 1991; 25: 248-250.
- Grivsky EM, Lee S, Sigel CW, Duch DS, Nichol CA. Synthesis and antitumor activity of 2, 4-diamino-6-(2, 5-dimethoxybenzyl)-5-methylpyrido [2,3-d] pyrimidine. J Med Chem 1980; 23: 327-329.
- Thompson AM, Bridges AJ, Fry DW, Kraker AJ, Denny WA. Tyrosine kinase inhibitors 7, 7-amino-4-(phenylamino)-and 7-amino-4-((phenylmethyl) amino) pyrido (4, 3-d) pyrimidines: a new class of inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor. J Med Chem 1995; 38: 3780-3788.
- Donkor IO, Klein CL, Liang L, Zhu N, Bradley E, Clark AM. Synthesis and antimicrobial activity of some 6, 7-annulated pyrido [2,3-d] pyrimidines. J Pharm Sci 1995; 84: 661-664.
- Pastor A, Alajarin R, Vaquero JJ, Alvarez-Builla J, Casa-Juana MF, Sunkel C, Priego JG, Fonseca I, Sanz-Aparicio J. Synthesis and structure of new pyrido [2,3-d] pyrimidine derivatives with calcium channel antagonist activity. Tetrahedron 1994; 50: 8085-8098.
- Agarwal A, Ashutosh R, Goyal N, Chauhan PMS, Gupta S. Dihydropyrido [2,3-d] pyrimidines as a new class of antileishmanial agents. J Bioorg Med Chem 2005; 13: 6678-6684.
- Monge A, Merino VM, Sanmartin C, Fernandez FJ, Ochoa MC, Berllver C, Artigas P, Alvarez EF. 2-arylamino-4-oxo-3, 4-dihydropyrido-[2,3-d] pyrimidines: synthesis and diuretic activity. Eur J Med Chem 1989; 24: 24-29.
- Zhang LJ, Yang MY, Sun ZH, Tan CX, Weng JQ, Wu HK, Liu XH. Synthesis and antifungal activity of 1, 3, 4-thiadiazole derivatives containing pyridine group. Lett Drug Des Discov 2014; 11: 1107-1111.
- Gomha SM, Abdel-aziz HM. Synthesis and antitumor activity of 1, 3, 4-thiadiazole derivatives bearing coumarine ring. Heterocycles 2015; 91: 583.
- Liu X, Shi Y, Ma Y, Zhang C, Dong W, Pan L, Li B, Wang Z. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1, 3, 4-thiadiazol-2-yl)cyclopropane carboxamides. Eur J Med Chem 2009; 44: 2782-2786.
- Gomha SM, Abdel-aziz HM, Khalil KD. Synthesis and SAR study of the novel thiadiazole-imidazole derivatives as a new anticancer agents. Chem Pharm Bull 2016; 64: 1356-1363.
- Gomha SM, Abdel-Aziz HA. Synthesis of new heterocycles derived from 3-(3-methyl-1Hindol-2-yl)-3-oxopropanenitrile as potent antifungal agents. Bull Korean Chem Soc 2012; 33: 2985-2990.
- Gomha SM, Khalil KD, El-Zanate AM, Riyadh SM. A facile green synthesis and anti-cancer activity of bis-arylhydrazononitriles, triazolo (5, 1-c) (1, 2, 4) triazine, and 1, 3, 4-thiadiazoline. Heterocycles 2013; 87: 1109-1120.
- Gomha SM, Riyadh SM. Synthesis under microwave irradiation of (1, 2, 4) triazolo (3, 4-b) (1, 3, 4) thiadiazoles and other diazoles bearing indole moieties and their antimicrobial evaluation. Molecules 2011; 16: 8244-8256.
- Yan SL, Yang MY, Sun ZH, Min LJ, Tan CX, Weng JQ, Wu HK, Liu XH. Synthesis and antifungal activity of 1, 2,3-thiadiazole derivatives containing 1, 3, 4-thiadiazole moiety. Lett Drug Des Discov 2014; 11: 940-943.
- Gomha SM, Salah TA, Abdelhamid AO. Synthesis, characterization and pharmacological evaluation of some novel thiadiazoles and thiazoles incorporating pyrazole moiety as potent anticancer agents. Monatsh Chem 2015; 146: 149-158.
- Gomha SM, Ahmed SA, Abdelhamid AO. Synthesis and cytotoxicity evaluation of some novel thiazoles, thiadiazoles, and pyrido [2,3-d] (1, 2, 4) triazolo (4, 3-a)pyrimidin-5(1H)-one incorporating triazole moiety. Molecules 2015; 20: 1357-1376.
- Ozdemir A, Turan-Zitouni G, Kaplanc ZA, Revial G, Guven K. Synthesis and antimicrobial activity of 1-(4-aryl-2-thiazolyl)-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives. Eur J Med Chem 2007; 42: 403-409.
- Turan-Zitouni G, Ozdemir A, Guven K. Synthesis of some 1((N, N-disubstitutedthio-carbamoylthio) acetyl)-3-(2-thienyl)-5-aryl-2-prazoline derivatives and investigation of their antibacterial and antifungal activities. Arch. Pharm 2005; 338: 96-104.
- Flora F, Hosni HH, Girgis AS. Novel bis (1-acyl-2-pyrazolines) of potential anti-inflammatory and molluscicidal properties. Bioorg Med Chem 2006; 14: 3929-3937.
- Mamolo MG, Zampieri D, Falagiani V, Vio L, Banfi E. Synthesis and antifungal activity of (+/-)-1-(5-aryl-3-pyridin-2-yl-4, 5-dihydro-pyrazol-1-yl)-2-imidazol-1-yl-ethanone derivatives. Farmaco 2003; 58: 315-322.
- Gomha SM, Riyadh SM, Abdalla MM. Solvent-drop grinding method: efficient synthesis, DPPH radical scavenging and anti-diabetic activities of chalcones, bis-chalcones, azolines, and bis-azolines. Curr Org Synth 2015; 12: 220-228.
- Shaharyar M, Siddiqui AA, Ali MM, Sriram D, Yogeeswari P. Synthesis and in vitro antimycobacterial activity of N1-nicotinoyl-3-(-4-hydroxy-3’-methyl phenyl)-5-((sub)phenyl)-2-pyrazolines. Bioorg Med Chem Lett 2006; 16: 3947-3949.
- Abid M, Azam A. Synthesis, characterization and antiamoebic activity of 1-(thiazolo(4, 5-b)quinoxaline-2-yl)-3-phenyl-2-pyrazoline derivatives. Bioorg Med Chem Lett 2006; 16: 2812-2816.
- Abid M, Azam A. 1-N-substituted thiocarbamoyl-3-phenyl-2-pyrazolines: synthesis and in vitro antiamoebic activities. Eur J Med Chem 2005; 40: 935-942.
- Ozdemir Z, Kandilci HB, Gumusel B, Calis U, Bilgin AA. Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur J Med Chem 2007; 42: 373-379.
- Amir M, Kumar H, Khan SA. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg Med Chem Lett 2008; 18: 918-922.
- Gomha SM, Riyadh SM, Mahmmoud EA, Elaasser MM. synthesis and anticancer activities of thiazoles, 1, 3-thiazines, and thiazolidine using chitosan-grafted-poly (vinylpyridine) as basic catalyst. Heterocycles 2015; 91: 1227-1243.
- Abdallah MA, Gomha SM, Morad MA, Elaasser MM. Synthesis of pyridotriazolopyrimidines as antitumor agents. J Heterocycl Chem 2016.
- Shawali AS, Gomha SM. A new entry for short and regioselective synthesis of (1, 2, 4) triazolo (4, 3-b) (1, 2, 4)-triazin-7 (1H)-ones Adv Synth Catal 2000; 342: 599-604.