Research Article - Biomedical Research (2017) Volume 28, Issue 5
Synthesis and in vitro evaluation of anti-breast cancer activity of a novel Dy (II) coordination polymer
Xiaowen Ding, Wenju Mo, Shuai Zhao, Hongjian Yang, Dehong Zou and Xiping Zhang*Department of Breast Tumor Surgery, Zhejiang Cancer Hospital, Hangzhou, Zhejiang, PR China
- *Corresponding Author:
- Xiping Zhang
Department of Breast Tumor Surgery
Zhejiang Cancer Hospital, PR China
Accepted date: September 21, 2016
Abstract
Discuss here is a kind of novel Dy-based Metal Organic Frameworks (MOF) material, ((Dy(H2O)(PBA) (SO4))(DMA)2(H2NC2H6))n(1 H2PBA=4-Hydroxybenzoic acid; the DMA=N,N-Dimethylacetamide), from the solvothermal synthesis conditions and using sulfuric acid as the PH controller and template. The X-ray studies shown that 1 is a 3D two-fold interpenetrated MOF built up from 1D-Dy (III) ribbon chains and PBA2- ligands. 1 title compound anti-breast cancer activity and organic ligand (H2PBA) survey of four kinds of human breast tumor cell line (BT474, ZR-75-30, MDA-MB-453 and Hs-578T) determined by MTT test. Found that compared with organic ligands, compound 1 exerted rather potent activity against all of these cell lines.
Keywords
Synthesis, X-ray, Anti-breast cancer
Introduction
In addition to surgery, radiotherapy and biological therapy, immune therapy and hormone therapy, treatment strategy for cancer treatment in clinical practice is currently focused on cytotoxic drugs as the main form of cancer chemotherapy [1,2]. Very heterogeneous compounds toxicity of cytotoxic drugs to treat cancer is mainly composed of rapid cell growth and division. Because cancer cells often experiencing rapid growth and spread, they are preferred by the agency [3-5]. The material is a traditional free drugs management solution in the form of intravenous injection. Although they use the long history and many more new drug development program to improve clinical success of treatment failure is often encountered, even in patients with malignant tumor is more sensitive to chemotherapy drugs (e.g., breast cancer), clinical results is usually lower than expected [6,7].
Coordination Polymers (CPs) is in the nature has not found another alternative mode. These compounds by the selfassembly of metal ions and organic ligands experiencing explosive growth because they are interesting topology and great potential applications in the field of luminescence, catalysis, magnetism, gas storage, etc. [8,9].
Biological activities of these compounds, however, have not been reported. In this work, we prepared a novel Dy-based MOF material, ((Dy(H2O)(PBA)(SO4)) (DMA)2(H2NC2H6))n(1 H2PBA=4-Hydroxybenzoic acid; the DMA=N,N-Dimethylacetamide), by using a linear organic link (Figure 1), and then to evaluate their antitumor activities.
Experimental
Apparatus and materials
All the starting materials and reagents used in this work for commercial and without purification before use. Elemental analysis (C, H, N) determination of element analyser Vairo EL III. Single crystal X-ray diffraction data records in mercury compound 1 CCD diffractometer. Melting point is XT-4 micro melting equipment, thermometer and uncorrected.
Synthesis and characterization of compound 1
A mixture of Dy(NO3)3·6H2O (0.045 g, 0.1 mmol), H2PBA (0.014 g, 0.1 mmol) and DMA (3 mL) were added to the 10 ml glass containers. Add 5 drops of sulfuric acid (20%, aq), glass container is closed and heated to 105°C to 72 h autogenous pressure. The ship was cooled to room temperature and yellow crystal. 1 yield is 32% (based on Dy(NO3)3·6H2O). Infrared (KBr, cm-1): 3225 (br), 1646 (s), 1382 (vs), 1229 (s), 862 (m), 835 (m), 656 (s), 566 (s). Elemental analysis (%) (C9H14DyO8SN): C, 23.56; H, 3.08; N, 3.05; Findings: C: 23.21; H: 23.21; N: 3.12.
Crystal structure determination
Structure of the computer control of mercury measurement (Charge-Coupled Device) CCD diffractometer and graphite-monochromated Mo-Kα radiation (λ=0.71073) at T=293 (2) K absorption correction using the SADABS program. Structure solved using direct method, and improved the matrix least square method using SHELXS F2-97 [10] package. The data and the structure improvement of crystal compound 1 summarized in Table 1.
| Formula | C9H14DyO8SN |
|---|---|
| Mr | 460 |
| Temperature/K | 293(2) |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a/Å | 7.6118(3) |
| b/Å | 20.0572(8) |
| c/Å | 6.4099(3) |
| α/° | 90 |
| β/° | 104.158(5) |
| γ/° | 90 |
| V/Å3 | 948.88(7) |
| Z | 4 |
| Dcalc/g/cm-3 | 2.889 |
| μ (Mo Kα)/mm-1 | 8.126 |
| θ range/° | 3.427 to 26.369 |
| Reflections collected | 4091 |
| No. unique data (R (int)) | 1930 [0.0246] |
| No. data with I ≥ 2σ(I) | 1748 |
| R1 | 0.0626 |
| ωR2(all data) | 0.1626 |
Table 1: Crystal data and structure refinements for compound 1.
In vitro anticancer activity
The feasibility of the four human tumor cell line (BT474, ZR-75-30, MDA-MB-453 and Hs-578T) was determined using the determined by MTT test. Cells of 70-80% confluency treatment of various concentrations of the synthesized compounds were 1% Dimethyl Sulfoxide (DMSO) as a negative control. After 48 h hatch 20 μl determined by MTT solution (5 mg/ml of PBS) add and hatch an additional 4 h. After that, the medium is inspiratory carefully, 150 μl DMSO added. Hatch 15 minutes later, the optical density measurement at 490 nm using FlexStation 3 desktop multimode standard instrument (molecular devices, USA). Data record and analysis to assess the effect of test material cell viability and depress growth.
Results and Discussion
Molecular structure
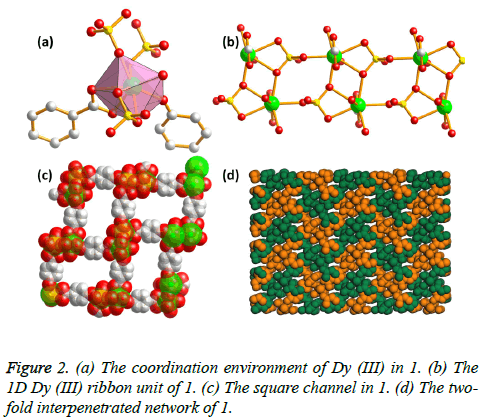
Single-crystal X-ray diffraction studies indicate that 1 crystallizes in the monoclinic space group P21/c, and a twofold interpeneted framework with 1D Dy (III) ribbon building units is presented. The asymmetric unit of 1 is composed of one Dy (III) ions, one PBA2- ligand, one SO42- ion, one coordinated water molecule and one disordered H2N(CH3)2 cation. The Dy (III) metal center is coordinated by eight O atoms from two PBA2- ligands, three SO42- ligands, and one coordinated water molecule, resulting in a [DyO8] coordinating configuration (Figure 2a). The Dy-O distances are in the range of 2.329 (7) to 2.514 (7) Å. Notably, two Dy (III) centers in the c axis are connected with each other via the SO42- ions, and such 1D chains are further inter-linked by the μ2-O atoms from the SO42- ions along a axis to afford an infinite Dy (III) ribbon building units (Figure 2b). These Dy (III) ribbons are connected by the PBA2- ligands to construct a 3D network with the distances between neighbouring Dy (III) ions in 4.049 Å. Such structural arrangements give large 1D channel with pore diameter ca. 8 Å (Figure 2c). However, due to the two-fold interpenetration, 1 is a dense structure with no residual solvent accessible area in unit cell (Figure 2d). From a topological view point, the structure can then be described as a new 3, 5- connected net by viewing the Dy ion as five connected node and the PBA2- ligand as a three-connected node, and the point symbol for this topology is (42.65.83) (42.6).
Antitumor activity
The cytotoxicity of synthetic compound 1 and organic ligand (H2PBA) on BT474, ZR-75-30, MDA-MB-453 and Hs-578T cell lines were determined by MTT test evaluation and IC50 value concentration from the experimental data are summarized in Table 2. Comparison, the purpose of a standard anti-cancer drug docetaxel is an assessment under the same conditions.
| Compounds | IC50 (μM) | |||
|---|---|---|---|---|
| BT474 | ZR-75-30 | MDA-MB-453 | Hs-578T | |
| Compound 1 | 18 | 20 | 32 | 22 |
| H2PBA | >100 | >100 | >100 | >100 |
| Docetaxel | 23 | 25 | 30 | 25 |
Table 2: Growth inhibitory effects of compound 1, organic ligand (H2PBA) and docetaxel on BT474, ZR-75-30, MDA-MB-453 and Hs-578T cell lines.
Obviously, organic ligand (H2PBA) is not active against all these cell lines (IC50>100 μM). In this concentration, organic ligands should exert high poisonous to the cells of normal cells, so we came to the conclusion that it did not show any living selectivity of these cell lines. However, tumor cells after incubation of compound 1 72 h IC50 value concentration of compound aged 18 to 32 μM, similar reference drug docetaxel, show title compound 1 showed antitumor activity to all of these cell lines to different degrees.
Conclusion
All in all, we have successfully synthesized a new Dy (III) based on the framework of infinite Dy (III) ribbon construction units according to a novel topology. Antitumor activity in vitro experiments show that the title compound 1 is a potential anticancer drug.
References
- Smith NF, Figg WD, Sparreboom A. Recent advances in pharmacogenetic approaches to anticancer drug development. Drug Develop Res 2004; 62: 233-253.
- Sloan KB, Wasdo S. Designing for topical delivery: prodrugs can make the difference. Med Res Rev 2003; 23: 763-793.
- Loncle C, Brunel JM, Vidal N, Dherbomez M, Letourneux Y. Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur J Med Chem 2004; 39: 1067-1071.
- Sridhar SK, Pandeya SN, Stables JP, Ramesh A. Anticonvulsant activity of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur J Pharm Sci 2002; 16: 129-132.
- Jarvest RL, Pinto IL, Ashman SM, Dabrowski CE, Fernandez AV. Inhibition of herpes proteases and antiviral activity of 2-substituted thieno [2,3-d] oxazinones. Bioorg Med Chem Lett 1999; 9: 443-448.
- Zhu W, Dai M, Xu Y, Qian X. Novel nitroheterocyclic hypoxic markers for solid tumor: synthesis and biological evaluation. Bioorg Med Chem 2008; 16: 3255-3260.
- Roberts LR, Gores GJ. Emerging drugs for hepatocellular carcinoma.Expert Opin Emerg Drugs 2006; 11: 469-487.
- El-Ayaan U, Abdel-Aziz AAM, Al-Shihry S. Solvatochromism, DNA binding, antitumor activity and molecular modeling study of mixed-ligand copper (II) complexes containing the bulky ligand: bis [N-(p-tolyl)imino]acenaphthene. Eur J Med Chem 2007; 42: 1325-1333.
- Zhao B, Cheng P, Dai Y, Cheng C, Liao DZ. A nanotubular 3D coordination polymer based on a 3d-4f heterometallic assembly.Angew Chem Int Ed Engl 2003; 42: 934-936.
- Sheldrick GM. SHELXL-97, Program for solution crystal structure and refinement. Univ Gottingen Germany 1997.