Review Article - Asian Journal of Biomedical and Pharmaceutical Sciences (2017) Volume 7, Issue 60
Synthesis and Biological Evaluation of 2-[(3-Cyano-1-Oxo-4-(3,4,5-Trimethoxyphenyl)-1,2,3,4-Tetrahydronaphthalen-2-Yl) Thio] Benzoic Acid Derivatives
Chaitramallu1, Devaraju Kesagodu1* and Ranjini2
1Department of Chemistry, Yuvaraja’s College, Mysore, India
2Department of Biotechnology, Maharani’s Science College, Mysore, India
- *Corresponding Author:
- Devaraju Kesagodu
Department of Chemistry
Yuvaraja’s College, Mysore, India
E-mail: malluchaithra88@gmail.com
Accepted on February 23, 2017
Abstract
A series of 2-[(3-cyano-1-oxo-4-(3,4,5-trimethoxyphenyl)-1,2,3,4-tetrahydronaphthalen-2-yl) thio] benzoic acid have been synthesized with a significant stereo selectivity and improved yields in a single step by employing thiosalicylic acid in presence of Tetra-n-butylammonium bromide/methanol solvent system. The structures of the synthesized compounds were confirmed by spectral and elemental analysis data. The synthesized compounds were screened for their biological activity
Keywords
Thiosalicylic acid, Tetra-N-Butylammonium bromide, Dehalogenation
Introduction
Podophyllotoxin (PPT, 1, Figure 1), a naturally occurring aryltetralin lignan, holds a unique position among natural products having been known for approximately 1000 years from its first application in folk medicines to its most recent developments in PPT-derived antitumor agents. Interest in PPT was initiated by Kaplan, who demonstrated its curative effect against tumor growth (Condylomata acuminata), and subsequently by King and Sullivan, who found its antiproliferative effect to be similar to that of colchicine at the cellular level [1]. But Due to its complicated side effects such as nausea, vomiting, and damage of normal tissues, attempts to use podophyllotoxin in the treatment of human neoplasia have been mostly unsuccessful. The unique cyclolignan scaffold of 1 has however drawn a lot of attention for the discovery and development of new anticancer agents. Extensive structural modifications, particularly at the C-4 and C-4′ position of podophyllotoxin have led to the development of many semisynthetic derivatives of podophyllotoxin. Among them, five semisynthetic derivatives, etoposide (2), teniposide (3), etopophos (4), GL-331 (5) and TOP-53 (6) (Figure 1) are currently used in the chemotherapy for a variety of cancers, including small-cell lung cancer, non-Hodgkin’s lymphoma, leukemia, Kaposi’s sarcoma, neurobslastoma and soft tissue sarcoma [2-4]. Their anticancer activity proceeds through a mechanism of action entirely different from that of their parent compound podophyllotoxin (1), Etoposide (2), teniposide (3), and etopophos (4) are three semisynthetic glucosidic cyclic acetals of 1, and in particular, etoposide (2) is considered to be one of the most successful pharmaceuticals derived from plants. Both GL-331 (5) and TOP-53 (6) are more active than etoposide (2) and are currently under clinical investigation [5-7].
Thus we describe here an efficient approach for the synthesis of 2-[(3-cyano-1-oxo-4-(3,4,5-trimethoxyphenyl)-1,2,3,4- tetrahydronaphthalen-2-yl) thio] benzoic acid derivatives as a key structural compound (Figures 2 and 3).
Results and Discussion
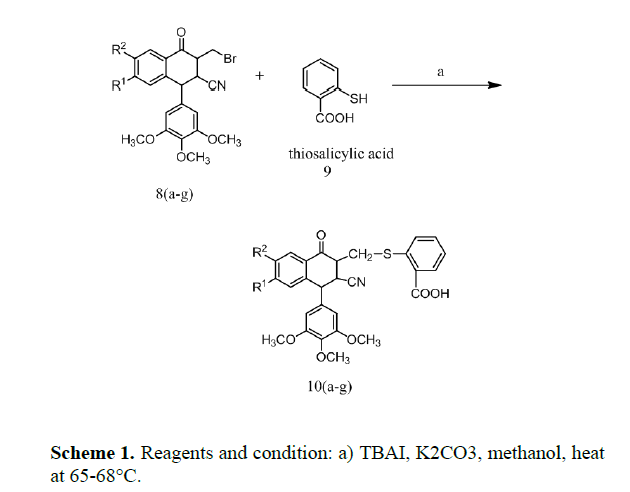
Substituted 2-[(3-cyano-1-oxo-4-(3,4,5-trimethoxyphenyl) -1,2,3,4-tetrahydronaphthalen-2-yl) thio] benzoic acid 10 a-g were prepared from substituted 3-bromomethyl-1- phenyl-1,2,3,4-tetrahydronaphthalene-2-carbonitrile 8 a-g (scheme 1) by substitution reaction with thiosalicylic acid using TBAI and potassium carbonate in methanol, stirred for 10 min at 25-28°C under nitrogen gas atmosphere and refluxed for 4-5 h at 65-68°C.After completion of the reaction, the reaction mixture was extracted with water followed by dichloromethane. The aqueous layer was collected and was evaporated to get crude solid which was purified by column chromatography on silica gel using hexane/ethyl acetate (80:20 v/v). Their structures were confirmed by spectroscopic evidences.
1H-NMR of the compound shows signals of a singlet at δ 11.0 indicating the presence of carboxylic group and a doublet at δ 4.00-4.05 for the proton bonding to cyanide group and sulphur group. 13C-NMR shows signals as singlet at 191.5 ppm pertaining to the carbonyl group, a singlet at 168.1 ppm for carboxylic acid and a singlet at 119.2 ppm for cyanide group.
2-((3-cyano-6,7-dimethoxy-1-oxo-4-(3,4,5- trimethoxyphenyl)-1,2,3,4 tetrahydronaphthalen-2-yl) thio) benzoic acid (10a)
1H NMR: 11.01-11.20(1 H, s, COOH), 8.30-8.35(1 H, d, Ar- H), 7.69-7.42(4 H, m, Ar-H), 7.07-6.89(s, 1H, Ar-H), 4.01-4.09(3 H, d, CH2), 3.92(15 H, s, OCH3), 2.79(2H, d CH2); 13C NMR: 191.5, 168.1, 154.7, 153.4, 147.2, 142.6, 137.3, 136.7, 134.1, 133.8, 133.2, 127.3, 126.7, 126.5, 125.0, 119.2, 110.5, 109.2, 106.6, 60.8, 56.1, 53.8, 36.6,31.6, 30.0; MS, m/z: 563.2 (M+). Anal. Calcd. For C30H29NO8 S: C, 63.93; H, 5.19; O, 22.71; S, 5.69; Found: C, 63.94; H, 5.18; O, 22.71; S, 5.68 %.
2-((3-cyano-6-hydroxy-1-oxo-4-(3,4,5- trimethoxyphenyl)-1,2,3,4 tetrahydronaphthalen-2-yl) methyl) thio) benzoic acid (10b).
1H NMR: 11.01-11.20(1 H, s, COOH), 8.30-8.19 (2 H, d, Ar- H), 7.69-7.42(3 H, m, Ar-H), 7.11(1 H, s, Ar-H), 6.89(s, 1H, Ar-H), 4.01-4.09(3 H, d, CH2), 3.92(9 H, s, OCH3), 2.79(2H, d CH2); 13C NMR: 191.5, 161.9, 168.1, 153.4, 142.6, 141.9, 137.3, 136.7, 134.1, 133.2, 130.7, 126.7, 126.6, 126.5, 125.0, 120.6, 113.3, 106.6, 60.8, 56.1, 53.8, 36.6,31.6, 30.0; MS, m/z: 519.10 (M+). Anal. Calcd. For C28H25NO7S: C, 64.73; H, 4.85; N, 2.70; O, 21.56; S, 6.17; Found: C, 64.72; H, 4. 86; N, 2.72; O, 22.55; S, 6.15%.
2-((3-cyano-6-methyl-1-oxo-4-(3,4,5- trimethoxyphenyl)-1,2,3,4-tetrahydronaphthalen-2- yl)methyl)thio)benzoic acid (10c)
1H NMR: 11.01-11.20(1 H, s, COOH), 8.35-8.30(1 H, d, Ar- H), 7.80-7.13(6 H, m, Ar-H), 7.07-6.89(s, 1H, Ar-H), 4.01-4.09(3 H, d, CH2), 3.92(9 H, s, OCH3), 2.79(2H, d CH2), 2.34(3 H, t, CH3); 13C NMR: 191.5, 168.1, 153.4, 143.3, 142.6, 140.4, 136.7, 134.1, 133.2, 131.0, 128.0, 126.7, 126.4, 125.2, 125.0, 119.2, 106.6, 60.8, 56.1, 53.8, 36.6, 31.6,30.0, 21.6; MS, m/z: 517.25 (M+). Anal. Calcd. For C29H27NO6S: C, 67.29; H, 5.26; N, 2.71; O, 18.55; S, 6.20; Found: C, 67.29; H, 5.25; N, 2.72; O, 18.57; S, 6.21 %.
2-((6-chloro-3-cyano-1-oxo-4-(3,4,5- trimethoxyphenyl)-1,2,3,4-tetrahydronaphthalen-2- yl)methyl)thio)benzoic acid (10d)
1H NMR: 11.01-11.20(1 H, s, COOH), 8.36-8.35(1 H, d, Ar- H), 7.86-7.39(6 H, m, Ar-H), 7.01(s, 1H, Ar-H), 4.01-4.09(3 H, d, CH2), 3.92(9 H, s, OCH3), 2.79(2H, d CH2); 13C NMR: 191.5, 168.1, 153.4, 142.6, 141.9, 139.2, 137.3, 136.7, 134.1, 133.2, 132.1, 130.7, 127.9, 126.7, 126.5, 126.2, 125.0, 119.2, 106.6, 60.8, 56.1, 53.8, 36.6, 31.6, 30.0;MS, m/z: 538.15 (M+). Anal. Calcd. For C28H24NClO6 S: C, 62.51; H, 4.50; Cl, 6.59; O, 17.84; S, 5.96; Found: C, 62.52; H, 4.51; Cl, 6.56; O, 17.85; S, 5.95 %.
2-((3-cyano-1-oxo-4-(3,4,5-trimethoxyphenyl)-1,2,3,4- tetrahydronaphthalen-2-yl) methyl)thio)benzoic acid (10e)
1H NMR: 11.01-11.20(1 H, s, COOH), 8.30-8.35(1H, d, Ar- H), 7.69-7.42(4 H, m, Ar-H), 7.07-6.89(s, 1H, Ar-H), 4.01-4.09(3 H, d, CH2), 3.92(9 H, s, OCH3), 2.79(2H, d CH2); 13C NMR: 191.5, 168.1, 154.7, 153.4, 147.2, 142.6, 137.3, 136.7, 134.1, 133.8, 133.2, 127.3, 126.7, 126.5, 125.0, 119.2, 110.5, 109.2, 106.6, 60.8, 56.1, 53.8, 36.6,31.6, 30.0; MS, m/z: 503.12 (M+).Anal. Calcd. For C28H25NO6S: C, 66.78; H, 5.00; O, 19.06; S, 6.37; Found: C, 66.77; H, 5.02; O, 19.07; S, 6.38%.
2-((3-cyano-6-methoxy-1-oxo-4-(3,4,5- trimethoxyphenyl)-1,2,3,4-tetrahydronaphthalen-2-yl) methyl)thio)benzoic acid (10f)
1H NMR: 11.01-11.20(1 H, s, COOH), 8.30-8.25(2 H, d, Ar- H), 7.69-7.42(3 H, m, Ar-H), 7.15(1 H, s, Ar-H),6.89(1 H, d, Ar-H), 6.69(s, 1H, Ar-H), 4.01-4.09(3 H, d, CH2), 3.92(12 H, s, OCH3), 2.79(2H, d CH2); 13C NMR: 191.5, 168.1, 165.5, 153.4, 142.6, 141.5, 137.3, 136.7, 134.1, 133.2, 130.3, 126.7, 126.5, 126.3, 125.0, 119.2, 111.7, 106.6, 104.6, 60.8, 56.1, 55.8, 53.8, 36.6,31.6, 30.0; MS, m/z: 533.25 (M+). Anal. Calcd. For C29H27NO7S: C, 65.28; H, 5.10; O, 20.99; S, 6.01; Found: C, 65.27; H, 5.16; O, 20.98; S, 6.03 %.
2-((6-amino-3-cyano-1-oxo-4-(3,4,5- trimethoxyphenyl)-1,2,3,4-tetrahydronaphthalen-2- yl)methyl)thio)benzoic acid (10g)
1H NMR: 11.01-11.02(1 H, s, COOH), 8.30-8.25(2 H, d, Ar- H), 7.69-7.28 (3 H, m, Ar-H), 7.09(1 H, s, Ar-H), 6.70(2 H, s, Ar-H), 6.30(2 H, s, NH), 6.27-6.24(3 H, t, Ar-H), 4.02-4.05(3 H, d, CH2), 3.93(9 H, s, OCH3), 2.79(2H, d CH2);13C NMR: 191.5, 168.1, 153.4, 153.3, 142.6, 141.3, 137.3, 136.7, 134.1, 133.2, 130.1, 126.7, 126.5, 125.0, 124.0, 119.2, 111.6, 115.1, 106.6, 60.8, 56.1, 53.8, 36.6,31.6, 30.0; MS, m/z: 519.15 (M+). Anal. Calcd. For C28H26N2O6S: C, 64.85; H, 5.05; O, 18.51; S, 6.18; Found: C, 64.87; H, 5.06; O, 18.50; S, 6.18 %.
Biological Assay
Anti-microbial activity
Antibacterial activity of the synthesized compounds with different concentrations of drug (20, 40, 80, 100 μg/disc) was determined against Gram-positive bacteria (Bacilus subtilius, Streptococcus) and Gram-negative bacteria (Escherichia coli, Proteus) in DMF by disc diffusion method on nutrient agar medium and also Anti-fungal activity of the synthesized compounds was determined against diploid fungus Candida albican. Gentamycin is used as positive control for comparison of anti-bacterial activity whereas Flucanozole is used as positive control for the comparision of anti-fungal activity. For each treatment, three replicates were maintained. The plates were incubated at 37°C for 24 h and the zone of inhibition was determined.
The results were compared with reference drugs and depicted in the above Table. The Table 1 reveals that 10 d, 10 e, 10 g, showed a potent anti-bacterial activity and 10g showed a potent anti-fungal activity of all the compounds which were under study with the MIC values ranging from 1.2 μg to 7 μg. Compounds 10b, 10c and 10f were not acting on Gram-positive bacteria B. subtilis. Compared to the reference compounds, the activity of compounds 10 d, 10 e, 10 g, 10 g, was significant whereas activities of rest of the compounds were not significant.
| Compounds | Minimum inhibitory concentration(µg) | ||||
|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | Proteus | C.albicans | |
| 10a | 3 | 3 | 3 | 3 | 3 |
| 10b | 3.5 | - | 3.5 | 4 | 5.5 |
| 10c | 4.0 | - | 3 | 5 | 2 |
| 10d | 0.3 | 0.72 | 1.8 | 1.4 | 1.3 |
| 10e | 0.4 | 0.75 | 1.3 | 1.3 | - |
| 10f | 1.5 | - | 2.5 | 4.5 | 2.5 |
| 10g | 0.5 | 0.75 | 1.1 | 1.2 | 0.95 |
Table 1. Anti-microbial activities of synthesized compounds 10(a-g)
DPPH radical scavenging assay
DPPH radical reacts with an Anti-oxidant compound that can donate proton and get reduced. DPPH when acted upon by an Anti-oxidant is converted into diphenyl picryl hydrazine. This can be identified by the conversion of purple to light yellow colour [8]. This can be quantified spectrophotometrically at 540 nm to indicate the extent of DPPH scavenging activity by the compounds. The radical scavenging activity was measured as the decrease in the absorbance of DPPH & calculated using the following equation
DPPH scavenging activity (%)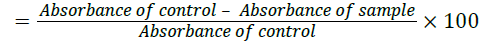
Where,
Absorbance of control=Absorbance of DPPH radical+ethanol
Absorbance of sample=Absorbance of DPPH radical+sample extract /standard.
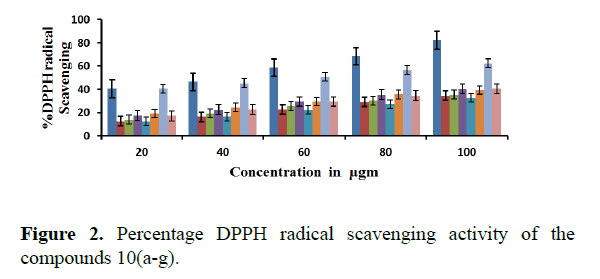
All the compounds of tetralin derivatives showed significant scavenging activity of the DPPH radicals compared to the reference compound BHT. The change in absorbance produced by reduced DPPH was used to evaluate the quenching ability of the synthesized compounds to act as free radical scavengers. DPPH decolorization was increased by the presence of 10 a, 10 b, and 10 g in a concentration dependent manner with an IC50 value of 18.12 μg/mL,19.67 μg/mL, 19.25 μg/mL and 18.15 μg/mL indicating the potent DPPH radical activity. DPPH decolorization was decreased by the presence 10f indicating less DPPH radical activity. The compounds 10c and 10e were found to have similar scavenging activity with an IC50 value of 22.5 μg/mL (Figure 1).
Alpha-amylase inhibition Assay
In this study, amylase activity for synthesized compounds was assayed according to Bernfeld method. This assay is based on the oxidation of ketone functional group in synthesized compounds. The principal involved is the test for the presence of free carbonyl group (C=O), so called reducing sugar. One mole of sugar will react with one mole of 3,5- dinitrosalicylic acid. Simultaneously 3,5-dinitrosalicylic acid (DNS) is reduced to 3-amino, 5-nitro salicylic acid under alkaline conditions [9].
The absorbance of the reaction mixture was read at 540 nm using UV spectrophotometer (Tables 2 and 3).
| Compounds | IC50 µg/ml |
|---|---|
| BHT | 37.4 |
| 10a | 18.76 |
| 10b | 21.75 |
| 10c | 22. 50 |
| 10d | 23.33 |
| 10e | 22.67 |
| 10f | 35.62 |
| 10g | 21.25 |
Table 2. Percentage IC50 values of 10(a-g) in DPPH radical scavenging activity.
| Compounds | IC50 in µg/mL |
|---|---|
| 20a | 32.25 |
| 20b | 21.27 |
| 20c | 33.70 |
| 20d | 44.37 |
| 20e | 40.69 |
| 20f | 45.38 |
| 20g | 43.70 |
| Acarbose | 95.80 |
Table 3. Pancreatic a-amylase inhibition of aryl tetralin derivatives 10(a-g).
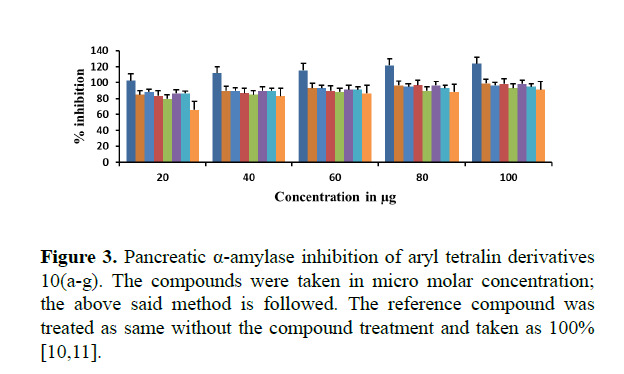
The Pancreatic α-amylase inhibition of synthesized compounds 10(a-g) were studied in terms of oxidation of ketone functional group using DNS method. All the compounds inhibited α- amylase enzyme significantly with an IC50 values ranging between 23.25 μg/mL and 46.5 μg/mL compared to the reference compound acarbose with an IC50 value of 95.8 μg/mL.
Conclusion
In summary, a facile and one pot synthesis of 2-[(3-cyano-1- oxo-4-(3,4,5-trimethoxyphenyl)-1,2,3,4- tetrahydronaphthalen-2-yl) thio] benzoic acid derivatives was achieved with an excellent yield. The synthesized compounds exhibited good antimicrobial, antioxidant and alpha amylase activities compared to the reference.
Acknowledgement
The authors are thankful to the University of Mysore, Mysuru for providing the spectral data.
References
- Liu YQ, Tian J, Qian K, Zhao XB, Morris-Natschke SL, Yang L, Nan X, Tian X, Lee KH. Recent progress on C-4-modified podophyllotoxinanalogs as potent antitumor agents. Med Res Rev. 2015; 35: 1-62.
- Zi CT, Xu FQ, Li GT, Li Y, Ding ZT, Zhou J, Jiang ZH, Hu JM. Synthesis and anticancer activity of glucosylatedpodophyllotoxin derivatives linked via 4Î?-triazole rings. Molecules. 2013; 18: 13992-14012.
- Jardine, Podophyllotoxins I. In Anticancer Agents based on Natural Product Models; Cassady JM, Douros JD, Eds.; Academic Press: New York, NY, USA, 1980.
- Gordaliza M, García PA, del Corral JM, Castro MA, Gómez-Zurita MA. Podophyllotoxin: Distribution, sources, applications and new cytotoxic derivatives. Toxicon. 2004; 44: 441-459.
- Terada T, Fujimoto K, Nomura M, Yamashita J, Wierzba K, Yamazaki R, Shibata J, Sugimoto Y, Yamada Y. Synthesis and biological activity of 4ß-alkyl derivatives containing hydroxy, amino, and amido groups of 4'-O-demethyl-4-desoxypodophyllotoxin as antitumor agents. J Med Chem. 1993; 36: 1689-1699.
- Xiao Z, Xiao YD, Feng J, Golbraikh A, Tropsha A, Lee KH. Antitumor agents. 213. Modeling of epipodophyllotoxin derivatives using variable selection k nearest neighbor QSAR method. J Med Chem. 2002; 45: 2294-2309.
- Tawa R, Takami M, Imakura Y, Lee KH, Sakurai H. Effects of CpG methylation to double stranded DNA breaks by Cu(II)-podophyllotoxin derivative complexes. Bioorg Med ChemLett. 1997; 7: 489-494.
- Kumar A, Illavarasan R, Jayachandran T, Deecaraman M, Aravindan P, Padmanabhan N, Krishnan MR, Anti-diabetic activity of Syzygiumcumini and its isolated compound against streptozotocin-induced diabetic rats. J Med Plant Res. 2008; 2: 246-249.
- Gandhimathi C, WC Sathiyasekaran B, Perumal PT, Rose C. Nutritional Evaluation, in vitro Free Radical Scavenging and in vivo Anti-inflammatory Effects of Gisekiapharnaceoides and Identification of Kaempferol as a Nutraceutical Agent. British Biotech J. 2011; 1: 113-135.
- Bernfeld P, Amylases: alpha and beta methods.Enzymol. 1955; 1: 149-158.
- Morris C, Fichtel SL, Taylor AJ. Impact of Calcium on Salivary a-Amylase Activity, Starch Paste Apparent Viscosity, and Thickness Perception. Cecile Chemosensory Perception. 2011; 4: 116-122.