Research Article - Biomedical Research (2017) Volume 28, Issue 7
Synthesis and anti-inflammatory activity in myocarditis of (2S, 5R)-6-(benzyloxy)-7-oxo-1, 6-diazabicyclo [3.2.1] octane-2-carboxamide
Xiao-Hui Liu1*, Yan Liu2 and Yu Zheng31Department of Ultrasound, Weinan Central Hospital, Weinan, Shannxi, PR China
2Department of Neurology, Weinan Central Hospital, Weinan, Shannxi, PR China
3Department of Ultrasound, Xi’an Central Hospital, Xi’an, Shannxi, PR China
Accepted on December 1, 2016
Abstract
The new heterocycles compound (2S, 5R)-6-(benzyloxy)-7-oxo-1, 6-diazabicyclo [3.2.1] octane-2- carboxamide, designed using (2S, 5R)-5-((benzyloxy) amino) piperidine-2-carboxylate oxalate as start material, was successfully obtained via multiple synthesis route and finally characterized by IR, 1H NMR, HRMS, and single crystal X-ray crystallography. The title compound was then screened for the in vivo anti-inflammatory property utilizing a standard acute carrageenan-induced paw oedema method in rats, and the compound showed promising activity.
Keywords
Heterocycles, Crystal, Anti-inflammatory.
Introduction
Myocarditis, also known as inflammatory cardiomyopathy, is inflammation of the heart muscle. Symptoms can include shortness of breath, chest pain, decreased ability to exercise, and an irregular heartbeat [1]. The duration of problems can vary from hours to months. Complications may include heart failure due to dilated cardiomyopathy or cardiac arrest [2]. In 2013, about 1.5 million cases of acute myocarditis occurred. While people of all ages are affected, the young are most often affected [3]. It is slightly more common in males than females. Most cases are mild. In 2015 cardiomyopathy, including myocarditis resulted in 354,000 deaths up from 294,000 in 1990. The initial descriptions of the condition are from the mid-1800s [4].
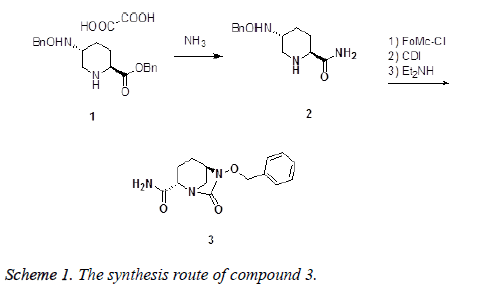
The progress achieved in the synthesis of heterocyclic compounds with biological potential is due to improvement of the methodological study of tested substances too [5]. It is known that many heterocyclic derivatives have biological activity, with their antibacterial, antimycobacterial, antimycotic, antifungal, antidepressive, and cardiotonic action being notable [6,7]. Recent research has also established for these heterocycles an analgesic and anti-inflammatory activity [8]. The present work deals with the synthesis and characterization of the title compound (2S, 5R)-6- (benzyloxy)-7-oxo-1, 6-diazabicyclo [3.2.1] octane-2- carboxamide (3). The compound (3) were synthesized by ammonolysis benzyl (2S, 5R)-5-((benzyloxy) amino) piperidine-2-carboxylate oxalate (1) as start material obtaining (2S, 5R)-5-[(benzyloxy) amino] piperidine-2-carboxamide (2), then treated with 9-fluorenylmethyl chloroformate, carbonyl diimidazole and diethylamine in one pot (Scheme 1). The antiinflammatory activity of the title compound (3) was then evaluated.
Experimental Methodology
Apparatus and materials
IR spectra (400-4000 cm-1) were obtained using a Brucker Equinox-55 spectrophotometer. 1H NMR spectra were obtained using a Varian Inova-400 spectrometer (at 400 MHz). Mass spectra were obtained using a micrOTOF-Q II mass spectrometer. The melting points were taken on a XT-4 micro melting apparatus, and the thermometer was uncorrected.
Synthesis and characterization of compounds 2 and 3
To a 250 ml three necked flask was added 70 ml ammonia in menthol (7 N) and 10 g compound 1 at room temperature. The mixture was stirred for 8 hours, and filtrated, the cake was washed by menthol and dumped. The filtrate was concentrated under reduced pressure and purified by flush chromatography yielding 5.8 g compound 2 as white solid. 1H-NMR (400 MHz, CDCl3), δ (ppm): 7.32-7.37 (m, 5H), 7.65 (s, 1H), 5.85 (s, 1H), 5.41 (s, 1H), 3.30-3.34 (dd, J=2.0, 12 Hz, 1H), 3.19-3.16 (dd, J=3.2, 10.4 Hz, 1H), 2.98 (m, 1H), 2.45-2.51 (dd, J=9.2, 11.6 Hz, 1H), 2.08-2.12 (m, 1H), 1.92-1.95 (m, 1H), 1.73 (s, 1H), 1.50-1.53 (m,1H), 1.28-1.30 (m, 1H).
To a 250 ml three necked flask equipped with ice-water bath, 5 g (20 mmol) of compound 2, 10 ml chlorobenzene and 2.7 g (21 mmol) ethyldiisopropylamine was added in proper order, then 5.4 g (21 mmol) 9-fluorenylmethyl chloroformate solution in 20 ml chlorobenzene was added drop wise at room temperature. The mixture was stirred at room temperature until compound 2 was consumed. Then 4.3 g (26 mmol) carbonyl diimidazole was added in proper speed to keep the temperature maintain at room temperature. The mixture was stirred further until the intermediate formed at last step consumed. Then 6.0 g (82 mmol) diethyl amine was added drop wise, and stirred until reaction was complete. The mixture was acidified by diluted hydrochloric acid, and the layers were separated. The aqueous phase was retraction with dichloromethane, and the organic phase were combined, and dried by Na2SO4. The solvent was removed reduced peruse, and purified by chromatographic column affording 4.5 g compound 3 as withe solid. 230-231°C. IR (KBr pellet cm-1): 3265, 1650, 1600 cm-1. 1H-NMR (400 MHz, CDCl3), δ (ppm): 7.35-7.46 (m, 6H), 7.30 (s, 1H), 4.90-4.97 (dd, J=11.2, 18 Hz, 2H), 3.68-3.70 (d, J=4.8, 1H), 3.63 (s, 1H), 2.91 (s, 2H), 2.04-2.08 (m, 1H), 1.82-1.85 (m, 1H), 1.61-1.65 (m, 2H). HRMS (ESI+): m/z: calculated for C14H17N3O3: 298.1162 [M+Na+]; found: 298.1134.
Crystal structure determination
Suitable single crystals of compound 3 were obtained by evaporation of chloroform solution. The diffraction data were collected on a Bruker Smart Apex CCD area detector using a graphite monochromated Mo Kα radiation (λ=0.71073 Å) at room temperature. The structure was solved by using the program SHELXL-97 [9] and Fourier difference techniques, and refined by full-matrix least-squares method on F2. All hydrogen atoms were added theoretically. Crystallographic data for compound 3 are listed in Table 1.
| Formula | C14H17N3O3 |
|---|---|
| Mr | 275.31 |
| Crystal system | Monoclinic |
| Space group | P21 |
| a/Å | 6.3438 (4) |
| b/Å | 8.2671 (5) |
| c/Å | 12.9776 (11) |
| α/° | 90 |
| β/° | 94.239 (7) |
| γ/° | 90 |
| V/Å3 | 678.75 (8) |
| Z | 2 |
| Dcalc/g.cm-3 | 1.347 |
| Μ (Mo Kα)/mm-1 | 0.097 |
| θ range/° | 2.92 to 26.36 |
| Reflections collected | 2960 |
| No. unique data (R (int)) | 2260 (0.0113) |
| No. data with I ≥ 2 σ (I) | 1988 |
| R1 | 0.0355 |
| ω R2 (all data) | 0.0813 |
Table 1. Crystal data, data collection and structure refinement of compound 3.
Results and Discussion
Molecular structure
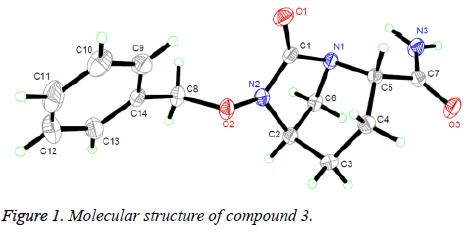
The structure of compound 3 was confirmed by 1H NMR and MS. These spectroscopic data are in good agreement with the assumed structure, and its structure was further determined by X-ray single-crystal diffraction analysis. The selected bond distances, bond angles and torsion angles are listed in Table 1.
The crystal structure of compound 3 is given in Figure 1. Geometric structure analysis of the molecule shows that the N (2)-C (1) (1.357 Å), N (1)-C (1) (1.443 Å) and N (3)-C (7) (1.331 Å) bonds are slightly shorter than the typical C-N bond (1.47-1.50 Å) [10] and normal N (1)-C (5) (1.497 Å) and N (1)-C (6) (1.498 Å) bonds. Such cases can be rationally explained that the lone-pair electrons in N atoms participate in forming π bonds with adjacent carbonyl group. The O (1)-C (1) in 1.207 Å is a double bond. The angles containing the unsaturated bond, O (1)-C (1)-N (1) and O (1)-C (1)-N (2), correspond to 124.09° and 128.62°, respectively. The piperazine ring, a non-planar six-membered heterocyclic ring, presents an ideal chair conformation. The bonds of C (5)-C (7) and N (2)-C (2) are in the vertical positions. The newly formed torsion angle of N (1)-C (1)-N (2)-C (2) is about 16.99°, indicating the plane exhibits nearly coplanar conformation, which is almost vertical to the main piperazine plane formed by atoms (C (5), N (1), C (2) and C (3)).
Anti-inflammatory activity
The anti-inflammatory activity of compounds 1-3 were evaluated by carrageenan induced paw oedema in rats, and the ED50 values derived from the experimental data were summarized in Table 2. As can be seen in Table 2, there is great difference in the anti-inflammatory activity between the three compounds. Compared with compounds 1 and 2, the title compound 3 showed more potent anti-inflammatory activity with ED50 value of 50.1 mg/kg, which is much lower than that of compounds 1 and 2.
| Compound | Anti-inflammatory activity (%) ± SME | |||
|---|---|---|---|---|
| Dose (mg/kg) | ED50 (mg/kg) | |||
| 25 | 50 | 100 | ||
| 1 | 2.8 ± 2.9 | 31.3 ± 1.8* | 45.5 ± 2.1 | 198.3 |
| 2 | 2.1 ± 2.5* | 27.3 ± 2.0 | 49.1 ± 2.5* | 187.3 |
| 3 | 26.1 ± 2.1 | 55.2 ± 3.0* | 72.1 ± 4.5 | 50.1 |
Table 2: Anti-inflammatory activity of compounds 1-3.
Anti-inflammatory activity by carrageenan induced paw oedema in rats at the end of 3 h. Drug was given orally as solution in distilled water (*p<0.05 Mann-Whitney test). Six rats were used for each test group and the control.
Conclusion
In conclusion, we synthesized a novel heterocycles derivative and characterized them via IR, 1H NMR, HRMS, and single crystal X-ray crystallography. From these data, we can conclude that compared with compounds 1 and 2, the antiinflammatory activity of the title compound 3 has been much improved. However, additional studies are needed to define the mechanism underlying its anti-inflammatory activity and evaluate the correlations of its drug efficacy in vivo.
References
- Zheng M, Zhou N, Ma L, Jin W. Inhibitory effects of the active constituents of ginseng stems and leaves on lung cancer NCI-H1650 cells. Biomed Res Ind 2015; 26: 646-650.
- Liu F, Wu Y, Li J, Zhou Y. Antitumor activities of pyran-annulated heterocycles. Lat Am J Pharm 2016; 35: 399-402.
- Biswas G, Sarkar S, Acharya K. Free radical scavenging and anti-inflammatory activities of the extracts of Astraeus hygrometricus (Pers.) Morg Lat Am J Pharm 2010; 29: 549-553.
- Chavan MJ, Wakte PS, Shinde DB. Analgesic and anti-inflammatory activities of saponified fraction from Annona reticulata L. Bark. Lat Am J Pharm 2010; 29: 1246-1249.
- Antonini I, Polucci P, Magnano A, Sparapani S, Martelli S. Rational design, synthesis, and biological evaluation of bis (pyrimido [5, 6, 1-de]acridines) and bis (pyrazolo (3, 4, 5-kl) acridine-5-carboxamides) as new anticancer agents. J Med Chem 2004; 47: 5244-5250.
- Akita M, Seto H, Aoyama R, Kimura J, Kobayashi K. Novel rearrangements in the reactions directed toward preparation of spiro-N, N-ketals: Reactions of naphthalene-1,8-diamine with ninhydrin and isatin. Molecules 2012; 17: 13879-13890.
- Girreser U, Heber D, Schutt M. 3-Benzoyl-3, 4-dihydro-2 H, 5 H-1-benzopyrano (4, 3-b) pyran-5-ones by condensation of 4-hydroxycoumarins with enone mannich bases. J Heterocyclic Chem 1998; 35: 1455-1460.
- Khlestkin VK, Tikhonov AY. Synthesis of pyrewmine and 1, 3-bishydroxylamine derivatives from enone Mannich base methiodides. Heterocyclic Communications 2002; 8: 249-254.
- Sheldrick GM. SHELXL-97, Program for Solution Crystal Structure and Refinement, University of Gotingen, Germany, 1997.
- Wilson JAC. International tables for crystallography. Kluwer Acad Publ Dordrecht the Netherlands 1992.