Research Article - Biomedical Research (2016) Volume 27, Issue 4
Synthesis and antidementia effects of a new Zn(II) coordination polymer
Chuan-Lai Han1, Rong Fu2 and Wei-Fu Lei1*
1Department of Anesthesiology, Qilu Hospital of Shandong University, Jinan, PR China
2Department of Anesthesiology, Nanjing Center Hospital, Nanjing, PR China
- *Corresponding Author:
- Wei-Fu Lei
Department of Anesthesiology
Qilu Hospital of Shandong University
Jinan
PR China
Accepted date: April 14, 2016
Abstract
A new 2D layered framework structure, namely, {[Zn(TPA)2](DMA)3}n (1, HTPA=4-(1,2,4-triazol-4-yl)- phenylacetic acid; DMA=N,N-Dimethylacetamide) were obtained under solvothermal conditions and structurally characterized. The X-ray studies shown that 1 possess a stair-shaped 2D (4, 4) network. In addition, cholinesterase inhibitory activities in vitro of the title compound and its corresponding organic ligand toward Glutaminyl cyclase (GC), Neprilysin (NeP) and Acetylcholine esterase (AChE) were further determined.
Keywords
Stair-shaped, X-ray, Cholinesterase.
Introduction
Alzheimer’s disease is a form of dementia that causes progressive changes in brain cells. It can occur in individuals as young as 40 years of age, but frequently occurs in those in their sixties [1,2]. The cause is unknown, but there are many theories currently being researched. A genetic defect, a missing enzyme, toxic effects of aluminium, a virus, and the faulty metabolism of glucose have all been implicated as possible causes [3,4]. Whatever the causes, Alzheimer’s disease is viewed as a terminal, incurable brain disease usually lasting from 3 to 10 years [5].
In recent years, the solid-metal supramolecular polymer has gradually become one of the most active research areas of chemical engineering and molecular sciences [6,7].
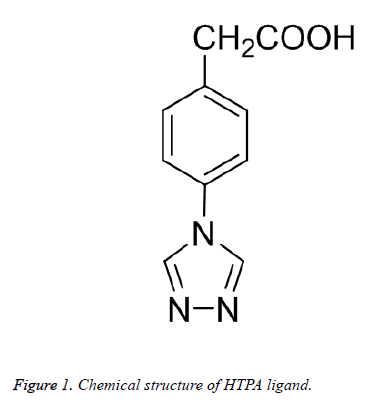
Furthermore, potential applications of supramolecular coordination compounds have been found in chemical sieving, sensing, and catalysis [8,9]. In this work, we prepared a novel stair-shaped 2D layered structure by using a mix-donor ligand (Figure 1), and then evaluated their cholinesterase activity.
Experimental
Apparatus and materials
All the starting materials and reagents used in this work were obtained commercially and used without further purification. Element analyses (C, H and N) were determined with an elemental Vairo EL III analyzer. Single-crystal X-ray diffraction data for compound 1 was recorded on Mercury CCD diffractometer. The melting points were taken on a XT-4 micro melting apparatus, and the thermometer was uncorrected.
Glutaminyl cyclase (GC), Neprilysin (NeP), Acetylcholine esterase (AChE) and butylthiocholine iodode (BTC) were obtained from Harbin Medical University. 5,5-Dithiobis-(2- nitrobenzoic acid) (DTNB), potassium dihydrogen phosphate, dipotassium hydrogen phosphate, potassium hydroxide, sodium hydrogen carbonate, and acetylthiocholine iodide were purchased from Nanjing Medical University.
Synthesis and characterization of compound 1
A mixture of Zn(NO3)2·6H2O (0.030 g, 0.1 mmol), HTPA (0.030 g, 0.15 mmol), DMA (3 mL) was added to a 20 mL glass vessel and heated to 90°C for 72 h under autogenous pressure. The vessel was then cooled down to room temperature and yellow block crystals were obtained. The yield was 80% for 1 (based on Zn(NO3)2·6H2O). IR (KBr, cm-1): 3421 (vs, br), 3100 (s), 1689 (s), 1672 (s), 1582 (m), 1526 (vs), 1311 (vs), 1201 (s), 1086 (s), 1021 (m), 853 (s), 764 (m), 669 (m). Elemental analysis calcd (%) for 1 (C20H16N6O4Zn): C, 52.57; H, 5.93; N, 17.24; found: C 52.21, H 5.67, N 17.12.
Crystal structure determination
Structural measurement was performed on a computercontrolled Mercury CCD diffractometer with graphitemonochromated Mo-Kα radiation (λ=0.71073 Å) at T=293 (2) K. Absorption correction was made using the SADABS program. The structure was solved using the direct method and refined by full-matrix least-squares methods on F2 by using the SHELXS-97 [10] program package. Crystallographic data and structural refinements for compound 1 are summarized in Table 1.
| Formula | C20H16N6O4Zn |
| Mr | 469.76 |
| Temperature/K | 293 (2) |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a/Å | 15.0010 (9) |
| b/Å | 10.9922 (6) |
| c/Å | 11.7719 (6) |
| α/° | 90 |
| β/° | 94.418 (5) |
| γ/° | 90 |
| V/Å3 | 1935.33 (19) |
| Z | 4 |
| Dcalc / g·cm-3 | 1.612 |
| μ(Mo Kα)/mm-1 | 1.312 |
| θ range/° | 3.472 to 25.00 |
| Reflections collected | 8739 |
| No. unique data [R(int)] | 3392 [0.0243] |
| No. data with I ≥ 2σ(I) | 2864 |
| R1 | 0.0465 |
| ωR2(all data) | 0.0995 |
| CCDC | 1451905 |
Table 1 Crystal data and structure refinements for compound 1.
Determination of inhibitory potency on GC, NeP, AChE
The inhibitory potency of target compounds on GC, NeP and AChE was determined using slightly modified Ellman’s method. In brief, 50 μL of five different concentration (10, 20, 30, 40, 50 μL) of the test compounds was added to the mixture of 3 mL phosphate buffer 0.1 M, pH=8.0 and 100 μL of DTNB solution. After 10 min of incubation at 25, 10 μL solution of acetylthiocholine iodide as substrate was added. The change of absorbance was measured at 412 nm for 6 min. The IC50 values were determined graphically from inhibition curves (log inhibitor concentration vs. percent of inhibition). The same method was used for GC and NeP inhibition assay.
Results and Discussion
Molecular structure
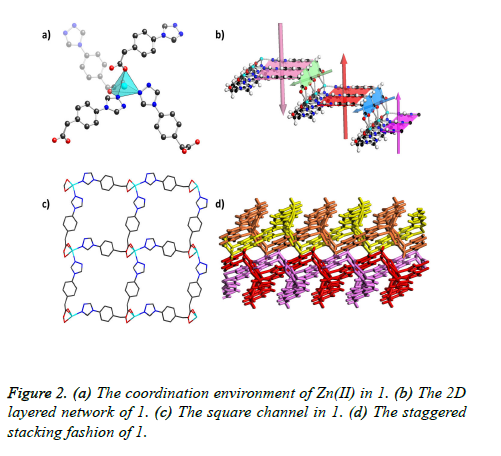
X-ray crystallography determination reveals that 1 crystallized in the monoclinic space group P21/c. The asymmetric unit of 1 comprises one Zn (II) ion and two TPA- ligands. The Zn (II) ion is five-coordinated with two triazol nitrogen atoms from two independent TPA- ligands, and the leaving three sites are finished by three O atoms from two carboxylic acid on another two independent TPA-ligands, forming distorted spherical square pyramid coordination geometry with a deviation of 3.775 as revealed by the shape software (Figure 2a). The Zn-N bond distances are in the range of 2.026(2)-2.041(2) Å, and the Zn-O bond distances are ranging from 1.912(2)-2.288(2). There are two TPA- ligands co-existing in the asymmetric unit with different coordination modes: one exhibits a twochelating mode with its one O atom and one N atom, the other possesses a three-chelating mode with its two O atom and one N atom. The TPA- ligands with the three-chelating mode join two adjacent Zn(II) ions along c axes to give rise to a 1D zigzag chain-lie structure (Figure 2b), which further extended to the 2D layered structure via the two-chelated TPA- ligands along b axes. All these connections lead to an interesting stairshaped 2D (4, 4) network that stacks in a parallel fashion with large square channels (Figure 2c). Due to its staggered stacking fashion, the framework of 1 is almost non-pore as calculated by software PLATON (Figure 2d).
Cholinesterase inhibitory activity
The title compound 1 and its corresponding organic ligand HTPA were evaluated for their in vitro inhibitory activities toward GC, NeP and AChE in comparison with commercially available donepezil as standard drug. The anti-cholinesterase activities are summarized in Table 2.
| Compound | GC (μM) | NeP (μM) | AChE (μM) |
|---|---|---|---|
| HTPA | 50.2 | 40.1 | 63.5 |
| 1 | 5.11 | 40.2 | 6.23 |
| Donepezil | 1.56 | 2.33 | 2.98 |
Table 2. GC, NeP and AChE inhibitory activities of compound 1, HTPA and donepezil.
The IC50 values in Table 2 revealed that compared with HTPA, compound 1 displayed higher GC and AChE inhibitory activity with the IC50 values of 5.11 and 6.23 μM. The organic ligand HTPA showed no inhibition on GC, NeP and AChE at concentrations less than 40.1-63.5 μM. Although the activity of compound 1 was less than standard drug donepezil (IC50=1.56, 2.33 and 2.98 for GC, NeP and AChE, respectively), but it had a fairly good inhibitory activity. Therefore, it could be considered as a new lead for further optimization.
Conclusion
In summary, a new Zn (II) coordination polymer, showing an interesting stair-shaped 2D (4, 4) network, was successfully obtained from organic ligand HTPA. From the experimental results, we can conclude that when the organic compound HTPA coordinated with Zn2+, the anti-cholinesterase activity of the title Ni (II) complex 1 has been much improved, which could be considered for Alzheimer’s disease therapeutics.
References
- Xu X, Park J, Hong YK, Lane AM. Synthesis and Characterization of Hollow Mesoporous BaFe12O19 Spheres. J Solid State Chem 2015; 222: 84-89.
- Rallas S, Gulerman N, Erdeniz H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5-disubstituted-1,3,4-oxadiazolines. Farmaco 2002; 57: 171-174.
- Gursoy A, Terzioglu N, Otuk G. Synthesis of some new hydrazide-hydrazones, thiosemicarbazides and thiazolidinones as possible antimicrobials. Eur J Med Chem 1997; 32: 753-757.
- Xu X, Park J, Hong YK, Lane AM. Magnetically Self-assembled SrFe12O19/FeCo Core/shell Nanoparticles. Mater Chem Phys 2015; 152: 9-12.
- Vicini P, Zani F, Cozzini P, Doytchinova I. Hydrazones of 1,2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 2002; 37: 553-564.
- Douillard S, Olivier D, Patrice T. In vitro and in vivo evaluation of radachlorin(R) sensitizer for photodynamic therapy. Photochem Photobiol Sci 2009; 8: 405-413.
- Xu X, Park J, Hong YK, Lane AM. Ethylene Glycol Assisted Spray Pyrolysis for the Synthesis of Hollow BaFe12O19 Spheres. Mater Lett 2015; 144: 119-122.
- Xu X, Hong YK, Park J, Lee W, Lane AM. Exchange Coupled SrFe12O19/FeCo Core/shell Composites with Different Shell Thickness. Electro Mater Lett 2015; 11: 1021-1027.
- Xu X, Hong YK, Park J, Lee W, Lane AM. Magnetic Self-Assembly for the Synthesis of Magnetic Exchange Coupled MnBi/FeCo Composites. J Solid State Chem 2015; 231: 108-113.
- Sheldrick GM. SHELXL-97, Program for Solution Crystal Structure and Refinement; University of Göttingen: Göttingen, Germany, 1997.