Research Article - Journal of Dermatology Research and Skin Care (2018) Volume 2, Issue 1
Survey on hereditary angioedema in a German cohort
- *Corresponding Author:
- Hahn J
Department of Oto-Rhino-Laryngology Head and Neck Surgery Ulm University Medical Center Frauensteige 12 89070 Ulm Germany
Tel: +49 731-50059501
E-mail: Janina.hahn@uniklinik-ulm.de
Accepted date: October 20, 2018
Citation: Hahn J, Hoess A, Schuler PJ, et al. Survey on hereditary angioedema in a German cohort. Dermatol Res Skin Care. 2018;2(1):6-11.
Abstract
Background and objectives: Hereditary angioedema (HAE) is a rare disease characterized by recurrent swelling attacks affecting almost every part of the body. So far, no reliable predictors for the clinical course have been identified. Here we evaluated the clinical situation of HAE patients in Germany and also aimed to analyze the role of body mass index (BMI) in disease severity.
Patients and methods: A survey among German HAE patients was done. The questions were related to the patients’ history, symptoms, and treatment. Groups with high and low BMI as well as different treatment modalities were compared.
Results: 61 patients were included. Fatigue proved the most frequent prodrome (37.7%), followed by psychiatric symptoms and skin changes. Swelling attacks predominantly affected the gastrointestinal tract, extremities and the head and neck region. About 60% of patients had at least monthly attacks. In most cases, first diagnosis was established by a dermatologist, followed by an ENT specialist and a general practitioner. Appropriate acute and prophylactic therapy reduced the number of days of sick leave by 81.3%. There was a tendency for a higher attack frequency in obese patients (BMI >30), but no statistically significant correlations could be established.
Conclusions: The majority of patients in this German cohort was provided state-of-the-art treatment, which lead to improved outcomes. Further studies assessing individual factors reflecting the disease severity like trigger factors or biomarkers may be considered.
Keywords
HAE, Attack frequency, Bradykinin, BMI, Days of sick leave, Prodromi.
Introduction
Hereditary Angioedema (HAE) is a rare disease with an incidence of about 1:50,000 [1]. According to current estimates prevalence of this autosomal dominant congenital disorder in Germany is roughly 1,600. As HAE is still relatively unknown and the symptoms are quite unspecific, resembling those of other diseases, a considerable number of unidentified cases is to be expected.
Due to a genetic variant HAE patients develop lifelong recurrent attacks of swellings, which may last several days if left untreated [2]. The edema may form in almost any part of the human body, predominantly the extremities and the digestive tract is affected [3]. A large part of the patients experiences prodromal symptoms like thirst or fatigue. There are also some well-known trigger factors like mechanical irritation and stress [4]. Interindividual variability in disease manifestation is huge, this including also attack frequency. Edema in the upper aerodigestive tract can be life threatening [5].
In most patients HAE is caused by a mutation of the “SERPING1” gene on the chromosome 11, which codes the C1 esterase inhibitor (C INH) [6]. C1 INH physiologically can implicate uncontrolled formation of the tissue hormone bradykinin. Accumulation of bradykinin can lead to edema development. The underlying gene defect can cause either a reduced C1 INH synthesis (HAE type 1, about 85% of patients) or the formation of a non-functional C1 INH (HAE type II, about 15% of patients) [7]. Rarely a third type is encountered, which was described simultaneously by Bork et al. and Binkely and Davis only in 2000. It is characterized by normal levels of functional C1 INH (in former times also called HAE type III) [8,9]. In some of these patients a mutation in the factor XII or the plasminogen coding gene can be detected [10,11].
The patient’s history including predominantly also the family history is mostly paving the way for suspecting HAE. However, there are de novo mutations in as many as a quarter of the patients, i.e. a negative family history does not rule out HAE [12].
The diagnosis is then based on the qualitative (=activity) and quantitative (=concentration) C1 INH values in blood plasma. In the typical HAE patient also the values of the C4 complement are too low [13]. The diagnosis of a HAE in case of normal C1 INH values is based on a positive family history in combination with the typical clinical signs. In some patients it can be further supported by the confirmation of a gene mutation.
In Germany there are two approved treatment options for HAE: Either substituting the missing or non-functional enzyme as intravenous C1 INH concentrate or controlling the bradykinin by subcutaneous injections of icatibant (investigational name HOE 140), which is a bradykinin-2-receptor antagonist [1,14]. Most of the therapeutic options are approved for home therapy by the patients or their relatives. Based on the attack frequency, the perceived burden of suffering and the potential trigger factors a short-term or long-term prophylaxis can be indicated in addition to acute treatment [15,16]. Unlike the more frequent mast cell mediated urticarial or allergic swellings, edema in HAE do not respond at all or only very little to antihistamines or corticosteroids [5]. Other drugs like kallikrein inhibitors were approved recently in the US, their market authorization for European countries is still pending.
The variability of the clinical course in combination with the unspecific symptoms and the scarce knowledge of the disease often results in a situation where the patients see many different specialty doctors until their suffering is finally diagnosed. This comprises general practitioners, dermatologists, ENT specialists and pediatricians. The time between the first symptoms and the correct diagnosis can be several decades and can include inadequate treatments based on false diagnoses [17]. Misdiagnoses associated the disease with the psychiatric field and led to the name “angioneurotic edema”, which was replaced by HAE after the identification of the underlying congenital defect.
Relatively little is known of the risk factors that may influence the course of the disease. The objectives of our survey study were to evaluate important parameters of HAE in Germany and to assess, if the BMI might have a role in disease severity.
Patients and Methods
This was a questionnaire-based study in adult patients with HAE (>18 years of age). The following parameters were addressedalso the effect of the introduction of adequate therapy was tried to assess retrospectively wherever possible:
• Demographic characteristics (age, sex).
• Height, weight, BMI.
• HAE type.
• Family history.
• Specialty responsible for making correct diagnosis.
• Prodromes.
• Attack sites.
• Frequency and duration of attacks.
• Days of sick leave.
• Medication.
After consultation with the statisticians and regarding the incidence of HAE, a minimum of 50 participants was targeted to obtain a realistic number and representative sample of patients with this rare disease.
We asked all HAE patients treated in our ENT department to participate. All patients were diagnosed by laboratory tests (levels of C1 INH protein <0.17g/l and/or dysfunctional C1 INH protein <70%). HAE with normal C1 INH was diagnosed when patients presented the above-mentioned clinical symptoms, had family members with history of angioedema and chronic urticaria was excluded. Patient questionnaires were collected between July 2015 and July 2016.
The evaluation of the questionnaires was done with descriptive statistics for all parameters. A potential correlation of BMI to attack frequency, location, duration of prodromes was statistically tested. Patients were assigned to two groups: Group 1=BMI <30 kg/m2; group 2=BMI ≥ 30 kg/m2. T-tests were carried out for these intergroup comparisons. A p-value of ≤ 0.05 was considered statistically significant, a p-value of ≤ 0.01 was considered highly significant. For all statistical evaluation the IBM SPSS Statistics 21 software was used. The study was approved by the review board of Ulm University (Application number 104/15).
Result
Demographic data
Filled in questionnaires from 61 patients were collected during the recruiting period. The mean patient age at inclusion was 43.2 years (range 20-74 years). 72.1% of the participants were female.
The average BMI of female participants was 24.9 ± 5.8 kg/m2, male participants had an average BMI of 26.9 ± 4.4 kg/m2. This means that both values are at the upper limit of normal weight, defined by the World Health Organization (WHO).
All patients were German nationals and living in Germany upon inclusion. Most of the patients came from southern Germany, which is due to the catchment area of the ENT clinic in Ulm.
Diagnostics: HAE type and family history
29 of the 61 participants (47.5%) were diagnosed as HAE type I, 21.3% as HAE type II, and 8.2% of the patients reported a diagnosis of HAE with normal C INH values. 14 patients (23.0%) did not know their diagnosed type of HAE.
44 patients (72.1%) stated that further family members were affected by HAE. In 41% of the patients only the ancestors were affected, in 18% of cases only the offspring had been diagnosed also with HAE, and in 13.1% of the participants the ancestors as well as the offspring was affected. 24.6% of all patients had no relatives with HAE.
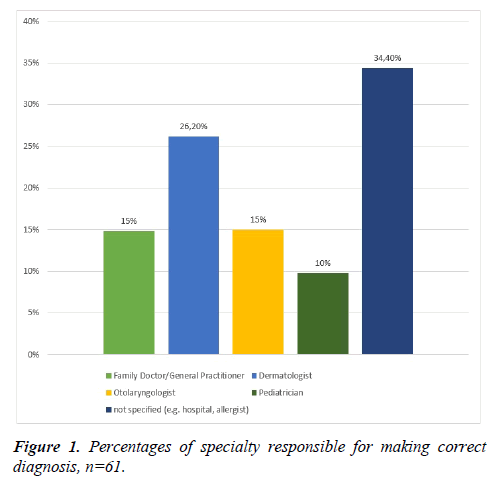
26.2% of the participants reported that their diagnosis was made by a dermatologist. 14.8% had been diagnosed by an ENT specialist or a general practitioner, respectively. 34.4% of the patients were unable to define what specialty doctor made their diagnosis but could state only more general aspects - e.g. that it was done in a hospital or by an allergist (Figure 1).
Symptoms
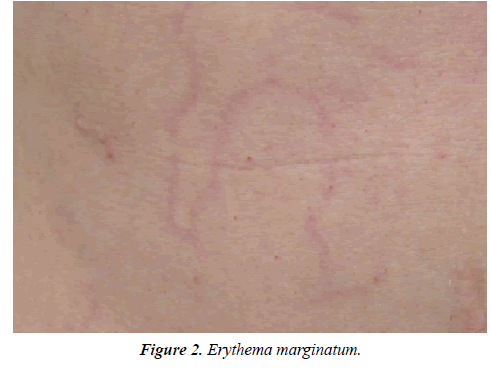
Fatigue was the most frequently reported prodrome before angioedema attacks in 37.7% of the patients. Psychiatric symptoms comprising mood swings, agitation, and irritability were stated by 18% of the HAE patients as prodromes. Among the 5 most frequently reported prodromes were also thirst, skin conditions like the Erythema marginatum (Figure 2) as well as paresthesia’s. Multiple mentions were possible for this question (Figure 3).
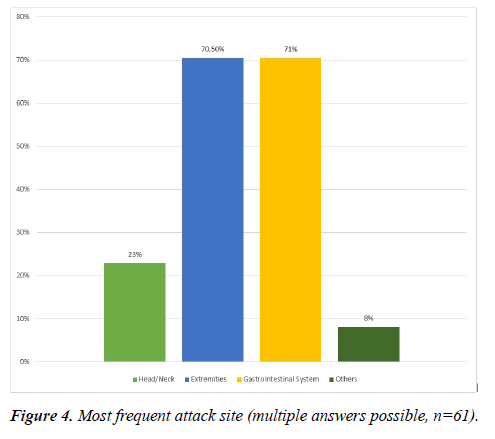
Also, regarding the affected locations in the body all answers could be checked that apply. The digestive tract and the extremities were reported most frequently by 70.5% of the patients. 23% of the patients stated that the head/neck region was among the predominantly affected parts of the body (Figure 4).
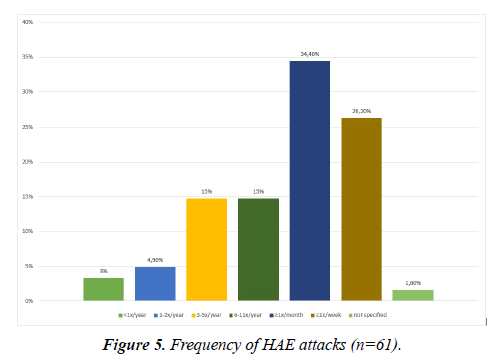
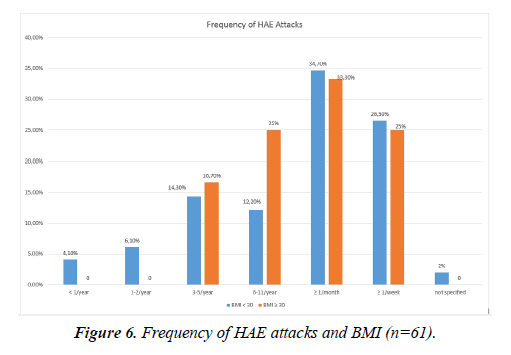
There was a high variability in the attack frequencies. 34.4% of the HAE patients reported to have acute swellings more than once a month but less than once a week. 26.2% suffered from swellings at least once a week. Only 2 patients (3.3%) reported swelling attacks less than once in a year (Figure 5). If left untreated, the edema lasted on average 3.8 (± 2.7) days. The duration ranged from a couple of hours to more than 10 days.
Therapy
The yearly mean number of days of sick leave from work or school was reported as reduced by 81.3% from 24 days without therapy to 4.5 days under adequate treatment.
47.5% of the HAE patients were prescribed a human or recombinant C1 INH concentrate for their acute therapy, 8% had C1 INH concentrate and Icatibant for acute therapy. 23% were treated only with the B2 antagonist icatibant for their acute swellings.
34.4% of the participants were on a prophylactic regimen. 32.8% used C1 INH concentrate, which is approved for prophylaxis. 1 patient used icatibant, which means an off-label use in Germany.
Correlation between BMI and disease severity
There were no significant correlations between the BMI and the duration or frequency of the attacks, the location of the swellings or the prodromes (p ≥ 0.05). There was a tendency towards a higher frequency of attacks in the HAE patients in the high BMI group (>30 kg/m2): All of them stated to have at least 3 attacks per year while 10.2% of the patients in the low BMI group reported to have less than 3 attacks in a year (Figure 6).
Discussion
Our prospective questionnaire based study evaluated clinical characteristics of HAE and the BMI as a potential risk factor for a more severe clinical course of this rare disease in a group of 61 adult German patients.
It was demonstrated that most of the patients were diagnosed by a dermatologist. In Germany there are currently seven large HAE centers, which are allocated to different medical specialties. They can be led by dermatologists, ENT specialists, internists, or pediatricians. The objective is, to allocate each HAE patient to one center that takes care of their individual treatment concept.
Further analyses of our questionnaire study targeted the more general course of the disease. The attack frequency has a major impact on the degree of suffering of the patients. Therefore, frequent attacks are a clear indication for starting a prophylactic treatment. In a Swiss study with 104 HAE patients a comparably broad range of attack frequencies was found. 39% of the HAE patients from Switzerland stated that they had an attack more often than once per month. Another study result were significantly higher frequencies of symptoms in female than in male HAE patients [18]. A study comprising Hungarian and Israeli children with HAE showed an even larger variability of the attack frequency than our study. 26.5% of the participants had 1-5 attacks per year, 23.5% reported 25-50 yearly attacks, and almost one third of the patients had been symptom free during the previous year. Also, the negative impact of a high attack frequency on the quality of life was shown in this study [19].
A large German study carried out by Magerl et al. studied the prodromes of HAE. It was shown, that about 79% of the 365 participating patients regularly suffered from prodromal symptoms. Fatigue, nausea, irritability, and skin conditions were the most frequently encountered signs, which is in line with our own results. Significantly more women than men reported to have prodromes in this German trial (83% vs. 73%; p<0.05) [20].
In our patient cohort HAE attacks most frequently affected the gastrointestinal tract and the extremities. This in alignment with the results from the Swiss study by Steiner et al., who found that the angioedema was localized predominantly in the abdomen (43% of cases), followed by swellings in the extremities [18]. Another German study by Bork et al. the affected parts of the body were divided into “affecting the skin” – including the extremities and the face, and other sites of manifestation like mucosa, or the internal organs. Out of the 209 HAE patients from this cohort, 100% reported skin manifestations, 97% gastrointestinal attacks, and 54% stated that they had swellings of the larynx [21]. Already in 2003 Bork et al. found a ratio of 1:70:54 for laryngeal to skin to abdominal attacks [22] The “Icatibant Outcome Survey” by Caballero et al. demonstrated 2017 that abdominal attacks predominated (58%) and they also showed, that the majority of attacks (90%) affect only a single anatomical site [23].
The therapy in our cohort proved to be in line with the treatment regimens recommended by the guidelines, which is based on C1 INH concentrates and bradykinin 2 receptor antagonists [24]. According to the individual clinical picture, acute medication schemes with or without additional prophylactic regimen were administered. The efficacy of a long-term treatment with C1 INH concentrates and the associated significant improvements in quality of life in HAE patients with frequent attacks was shown in several studies [15,16,25].
Consistent results from several studies showed that HAE attacks can be triggered by hormones like estrogen and that extraovarian estrogen is predominantly synthesized in visceral fat tissue by the aromatase cytochrome p450 [26,27]. Therefore, we studied a potential correlation between the BMI of the HAE patients with their clinical course. While we found a tendency towards a higher attack frequency in the obese patients, there were no statistically significant differences found in any parameter for the sub-group with a BMI ≥ 30 kg/m2 when compared to those with a BMI <30 kg/m2. There is a paucity of data regarding obesity as a risk factor in HAE. Bernstein et al. studied if a standardized, weight adjusted dosing of 20 IU/kg of C1 INH concentrate is effective in obese patients. The medication was found equally effective and safe in all patients, irrespective of their BMI [28]. As of now, no biomarker has been identified, that correlates with the individual clinical course of the disease – e.g. with the attack frequency. Not even fundamental factors like the type of HAE, the C1 INH activity, or the underlying mutation are no reliable predictors for the severity of the disorder. Current basic research studies target to find biomarkers, predictors, and predisposing factors for swelling attacks, in order to better understand this individually so variable disease and to then be able to treat more specifically [29,30].
One general limitation of our study is the questionnaire-based set-up. However, this scenario allowed for reaching a relatively large patient group in a short period of time and to provide the patient perspective directly. Another draw-back is the retrospective assessment of some parameters like the days of sick leave before the introduction of adequate therapy. While no accurate numbers can be expected, we believe that the gross picture of the situation is reflected to a sufficient extent.
Conclusion
The therapeutic options based on a better understanding of the disease HAE made considerable progress over the past decades. But especially concerning the individual differences of HAE patients, many issues are still unresolved. With future research on the clinical course, fundamental scientific aspects and carefully adjusted therapy, we hope that patients with the incurable disease HAE may be able to lead an almost normal life. Further studies assessing individual factors reflecting the disease severity like trigger factors or biomarkers may be considered.
Acknowledgement
JH, TKH and JG designed the study and the questionnaire and wrote the manuscript. JH, PJS and AH edited data and figures, AH and BM developed and performed the statistical analysis. All authors read and approved the final manuscript.
Conflict of Interests
JH, TKH and JG report non-financial support from Shire Deutschland GmbH and CSL Behring outside the submitted work. BM, AH and PJS report no conflicts of interest.
References
- Caballero T, Prior N. Burden of illness and quality-of-life measures in angioedema conditions. Immunol Allergy Clin North Am. 2017;37(3):597-616.
- Lumry WR. Overview of epidemiology, pathophysiology, and disease progression in hereditary angioedema. Am J Manag Care. 2013;19:s103-10.
- Hofman ZL, Relan A, Hack CE. Hereditary angioedema attacks: Local swelling at multiple sites. Clin Rev Allergy Immunol. 2016;50(1):34-40.
- Caballero T, Maurer M, Longhurst HJ, et al. Triggers and prodromal symptoms of angioedema attacks in patients with hereditary angioedema. J Investig Allergol Clin Immunol. 2016;26(6):383-6.
- Bernstein JA, Cremonesi P, Hoffmann TK, et al. Angioedema in the emergency department: A practical guide to differential diagnosis and management. Int J Emerg Med. 2017;10(1):17-41.
- Germenis AE, Speletas M. Genetics of hereditary angioedema revisited. Clin Rev Allergy Immunol. 2016;51(2):170-82.
- Zuraw BL. Clinical practice: Hereditary angioedema. N Engl J Med. 2008;359(10):1027-36.
- Binkley KE, Davis A. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema (3rd ed.). J Allergy Clin Immunol. 2000;106(3):546-50.
- Bork K, Barnstedt SE, Koch P, et al. Hereditary angioedema with normal C1-inhibitor activity in women. Lancet. 2000;356(9225):213-17.
- Cichon S, Martin L, Hennies HC, et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 2006;79(6):1098-104.
- Bork K, Wulff K, Steinmuller-Magin L, et al. Hereditary angioedema with a mutation in the plasminogen gene. Allergy. 2018;73(2):442-50.
- Pappalardo E, Cicardi M, Duponchel C, et al. Frequent de novo mutations and exon deletions in the C1 inhibitor gene of patients with angioedema. J Allergy Clin Immunol. 2000;106(6):1147-54.
- Aabom A, Bygum A, Koch C. Complement factor C4 activation in patients with hereditary angioedema. Clin Biochem 2017;12.
- Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med. 2010;363(6):532-41.
- Greve J, Strassen U, Gorczyza M, et al. Prophylaxis in hereditary angioedema (HAE) with C1 inhibitor deficiency. J Dtsch Dermatol Ges. 2016;14(3):266-75.
- Craig T, Shapiro R, Vegh A, et al. Efficacy and safety of an intravenous C1-inhibitor concentrate for long-term prophylaxis in hereditary angioedema. Allergy Rhinol (Providence). 2017;8(1):13-9.
- Martinez-Saguer I, Escuriola Ettingshausen C. Delayed diagnosis of hereditary angioedema: Thirty-nine years of inadequate treatment. Ann Allergy Asthma Immunol. 2016;117(5):554-6.
- Steiner UC, Weber-Chrysochoou C, Helbling A, et al. Hereditary angioedema due to C1 - inhibitor deficiency in Switzerland: Clinical characteristics and therapeutic modalities within a cohort study. Orphanet J Rare Dis. 2016;11:43016-4231.
- Engel-Yeger B, Farkas H, Kivity S, et al. Health-related quality of life among children with hereditary angioedema. Pediatr Allergy Immunol. 2017;4.
- Magerl M, Doumoulakis G, Kalkounou I, et al. Characterization of prodromal symptoms in a large population of patients with hereditary angio-oedema. Clin Exp Dermatol. 2014;39(3):298-303.
- Bork K, Meng G, Staubach P, et al. Hereditary angioedema: New findings concerning symptoms, affected organs, and course. Am J Med. 2006;119(3):267-74.
- Bork K, Hardt J, Schicketanz KH, et al. Clinical studies of sudden upper airway obstruction in patients with hereditary angioedema due to C1 esterase inhibitor deficiency. Arch Intern Med. 2003;163(10):1229-35.
- Caballero T, Aberer W, Longhurst HJ, et al. The Icatibant outcome survey: Experience of hereditary angioedema management from six European countries. J Eur Acad Dermatol Venereol. 2017;31(7):1214-22.
- Maurer M, Magerl M, Ansotegui I, et al. The international WAO/EAACI guideline for the management of hereditary angioedema: The 2017 revision and update. Allergy. 2018;10.
- Greve J, Hahn J, Nordmann M, et al. Nano-filtrated C1-esterase-inhibitor in the prophylactic treatment of bradykinin-mediated angioedema. Transfusion. 2016;56(5):1022-29.
- Caballero T, Farkas H, Bouillet L, et al. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol. 2012;129(2):308-20.
- Simpson ER, Merrill JC, Hollub AJ, et al. Regulation of estrogen biosynthesis by human adipose cells. Endocr Rev. 1989;10(2):136-48.
- Bernstein JA, Machnig T, Keinecke HO, et al. The effect of weight on the efficacy and safety of C1 esterase inhibitor concentrate for the treatment of acute hereditary angioedema. Clin Ther. 2014;36(4):518-25.
- De Maat S, Bjorkqvist J, Suffritti C, et al. Plasmin is a natural trigger for bradykinin production in patients with hereditary angioedema with factor XII mutations. J Allergy Clin Immunol. 2016;138(5):1414-23.
- Loffredo S, Bova M, Suffritti C, et al. Elevated plasma levels of vascular permeability factors in C1 inhibitor-deficient hereditary angioedema. Allergy. 2016;71(7):989-96.