Research Article - Biomedical Research (2017) Volume 28, Issue 8
Study on the preparation of spectral standard samples for cathode copper
Yansong Li1,2, Yang Hongying1* and Dawei Wang2
1School of Materials and Metallurgy, Northeastern University, Shenyang, Liaoning, P.R. China
2Shenyang Research Institute of Nonferrous Metals, Shenyang, Liaoning, P.R. China
- *Corresponding Author:
- Yang Hongying
School of Materials and Metallurgy
Northeastern University, PR China
Accepted on December 26, 2016
Abstract
Spectral cathode copper standard samples were prepared in the intermediate frequency induction furnace by adding necessary additives into 5N-level cathode copper. The manufacturing process, including design of the component, selection of raw materials, characterization of homogeneity, analysis of standards, statistical treatment of data and confirmation of values and standards deviation are presented in this manuscript. The effects of the preparation technological parameters on the sample variance were studied and the optimal processing conditions were obtained as follows: the casting temperature is 1200°C, the stirring rate is 120 rpm, the stirring time is 1 min, the heat preservation period is 25 min and argon dosage is 0.4 m3/kg. The spectral standard sample for cathode copper obtained under these conditions has a preferred orientation on the (220) plane and has been approved as a national level standard sample (No. GSB 04-2707-2011) by China National Standard Sample Technical Committee due to its compact and uniform structure, good stability, high accuracy and reliability.
Keywords
Standard sample, Cathode copper, Spectrum, Preparation process.
Introduction
Cathode copper is playing a very important role in chemical industry, defense industry and the construction industry [1-4], for example highly purified copper is widely used in network connection wires, electric vacuum components and superconductors. Thus it is a great challenge and urgent to get highquality cathode copper, especially in the field of accurately detecting the chemical composition of the cathode copper [5-9]. The emission spectrometry method is an effective way to detect the chemical components of the cathode copper [10-15], using the cathode standard samples as the standard substance. While the preparation of the standard samples is a very complex process, we need to add 18 kinds of impuritys elements including volatile and oxidable Cd and Zn, easily-loss elements such as Si, Zn and Mn and high melting point elements such as Ni and Fe. The content of every kinds of element should be in consistent with the designed value and distributed evenly, what’s more, the materials adding time, melting condition and materials adding order are also important factors that should be taken into consideration. Thus it is a very difficult process to prepare the standard samples.
Copper electrolysis refining method is a method using anodic copper (impurity: 99.0%~ 99.8%) casted into anode plates, pure copper as cathode, and the aqueous of sulfuric acid and cupric sulfate as electrolyte. Under the effect of direct current (dc), the anode copper and the potential negative base metals are dissolved into the solution, while precious metals and some insoluble metals turn into anode slime and sink in the bottom of the cell. As the electrolysis continues, copper in the solution preferentially electrodeposits on the cathode, and then more than 99.99% of high purity cathode copper could be obtained [6,16-17]. And other potential negative metals won’t be electrodeposited at the cathode in the electrolyte, thus can only be removed from the base by purification on a regular basis [18-21]. In the past years, the cathode copper standard sample must be from foreign imports with no authorities recognized. The preparation of cathode copper spectrum standard samples is seldom reported in domestic and foreign countries. On this basis, we apply to the national standard sample committee for project. Through the chemical composition design, material selection, the determination of optimum condition of the examination of the smelting, processing and evenness, the constant value and so on, we finally successfully developed cathode copper spectrum standard samples. This standard sample has reasonable chemical composition design, good uniformity and accurate fixed value. The standard sample has been approved by the national standard sample technical committee as the national standard samples with a standard number is GSB 04-2707-2011, and was awarded the national invention patent with patent number ZL 2013 1 0489178.2.
Design of component
According to the rule in "Working guidelines for standard sample" (GB/T15000) [22] and “technical specifications of standard samples used in the field of non-ferrous metal products analysis(YS/T409-1998) [23], the component design should cover and adapt to the technical conditions of products, meet the requirements of the analysis. After researching the national standard grades [24] of cathode copper and investigating the user's actual demand, we designed the chemical composition of the cathode copper standard samples with 18 species of selected elements content ranging from 0.0001% to 0.008% gradient distribution.
Material and Methods
Equipment
A medium-frequency induction furnace: Smelting quantity, 100 kg; the maximum melting temperature 2300°C; Photoelectric direct reading spectrometry (ARL4460).
Materials
The main material for the standard sample was high-pure copper with purity of 99.999 wt.%, and other given impurity elements with a purity of more than 99.99%, and the P was added by adding P2O5.with purity higher than 98.5%.
Preparation of cathode copper sample
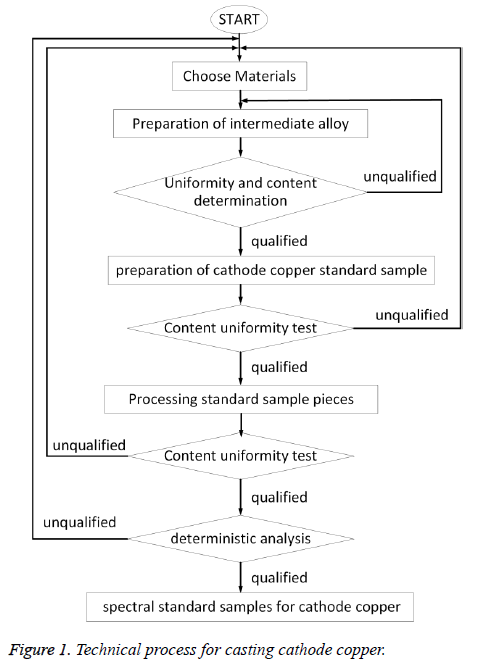
The cathode copper sample was prepared by the following two steps: (1) Preparation of copper master alloys. After oven operation, the high-pure copper was added and the charcoal was covered, and then the furnace temperature was elevated to the pre-set temperature. After this, 18 kinds of impurity elements were added in groups, the argon gas was introduced as shielding gas, and continuous stirring was kept until the form of copper alloys. (2) Prepare copper standard samples. Number them from 1 to 5. The amount of copper alloy was calculated according to the designed dosage of standard sample, then was added in group to the pure copper according to the experimental conditions, taking them out after melting, natural cooling, then processed them into standard samples, after examination of the homogeneity, setting value, then qualified cathode copper standard sample can be got. The preparation process was showed in Figure 1.
Analysis of the cathode copper standard samples
The cathode copper standard samples prepared in Step 2.3 was analyzed by X-ray diffract meter system (XRD, D8 Advance, German Brux) to test its crystal index, and the scanning electron microscope (SEM, JSM-5601LV, Japan) was used to observe the surface topography of the catalyst, and the relative content on the surface of the catalyst was test by Energy Dispersive Spectrometer (EDS). In addition, the transmission electron microscope (TEM, JEM-2100, Japanese Electron Company) was introduced to observe the homogeneity of the component.
Results and Discussion
It is well known that the two principles in the preparation of standard sample are: (1) The content of impurity elements should be consistent with the pre-planned value and the point value is gradient distributed; (2) Each element in the standard sample should be evenly distributed. Due to the fact that the different impurity elements had different waste percentage in the process of casting, thus we have divided the impurity elements into 4 groups, made into four groups of intermediate alloy, determine the element content, then made into standard samples.
The determination of the casting temperature
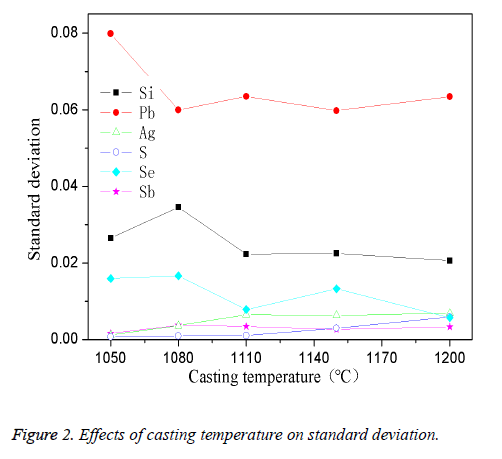
The high-pure copper was divided into several parts and put into the intermediate frequency induction furnace which was covered by charcoal. The planned quantity of other elements was added when the reaction temperature was reached. The mixture was stirred for 4 min while taking argon as shielding gas and then preserved for 35 min. The effect of casting temperature on standard deviation was showed in Figure 2.
As shown in Figure 2, under casting temperature of 1050°C, the standard deviation was high, especially for the element Pb, Si, Se; the standard deviation increased with the increase of temperature and reached a stable value when it reached 1200°C. When the temperature was too low (under 1050°C), the copper couldn’t be completely molten, the liquidity of the melt was poor, so the impurities in the melt were unevenly scattered. The uneven distribution of the impurities in the sample caused a higher element standard deviation. With the casting temperature gradually increased, the melt turned to be good flow characteristics, the impurities in the casting process can fully dispersed, and apparently impurity element standard deviation is smaller. Thus, the appropriate casting temperature is determined to 1200°C in this experiment.
The determination of the stirring speed
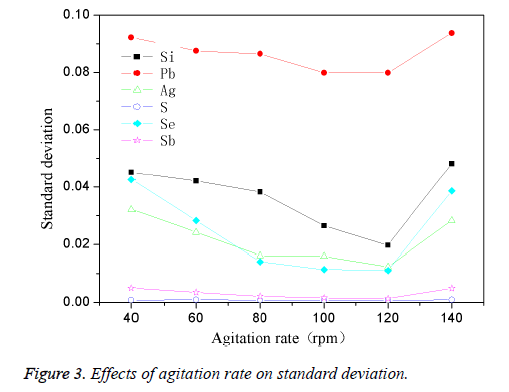
The high-purity copper was divided into several parts and put into the intermediate frequency induction furnace which was covered with charcoal. Then add in planned quantity of other element when the reaction temperature reached 1200°C. The mixture was stirred for 4min while taking argon as shielding gas, then preserved for 35 min. The effect of different stirring speed on standard deviation was shown in Figure 3.
Figure 3 showed that the standard deviation of the different elements was easily affected by stirring speed, when the stirring speed was 40 rpm, the standard deviation for most of the elements was larger; with the continuing increase of the stirring speed, standard deviation gradually decreased, and reached the maximum when stirring speed reached to 120 rpm. Continuing increasing the stirring speed, the standard deviation fluctuated significantly, which could be easily observed from the curves of Si, Pb, Ag and Se. This was because that when the stirring speed was too low (40 rpm), the speed of dispersion and eutectic of the impurity elements in copper melt slow, that caused the uneven distribution of the impurities in samples, then element standard deviation would be higher. When the stirring speed was accelerated, the dispersion speed and melting rate was accelerated, the impurities in the melt could be dispersed more evenly, thus we could get a more evenly dispersed sample with a lower standard deviation. However, when the stirring speed was too fast (140 rpm), the impurity elements and copper melt may sputter during the melt eutectic process, and volatilized fast at the same time, thus led to unknown amount of actual existence of impurity elements in the melt, then the standard sample would be in low homogeneity. Thus the optimum stirring speed was 120 rpm.
The determination of the stirring time
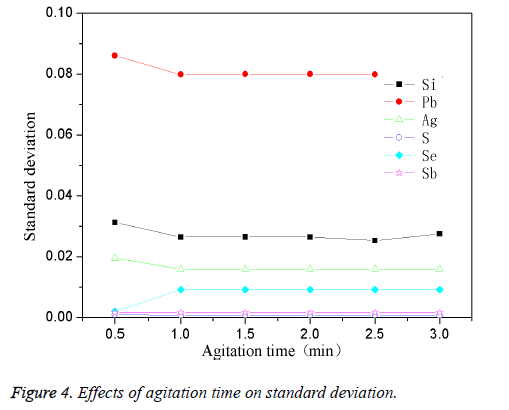
The high-purity copper was divided into several parts and put it into the intermediate frequency induction furnace which was with charcoal. The planned quantity of other elements was added when the reaction temperature reached 1200°C. The mixture was stirred for 1 min with a fixed stirring speed (120 rpm). The effect of different stirring time on standard deviation was shown in Figure 4. From Figure 4, it could be observed that the stirring time had less effect on standard deviation of the elements, when kept stirring for 0.5 min, the standard deviation for most of the elements were larger; while extending the stirring time to 1 min, the standard deviation could gradually change to be stable. This was because that when the stirring time was short (0.5 min), the impurity elements couldn't be mixed evenly with the copper melt, so the standard deviation was higher. After extending the stirring time, the two could be mixed more and more fully, thus a lower standard deviation could be obtained. Thus the optimum mixing time was 1 min.
The determination of the holding time
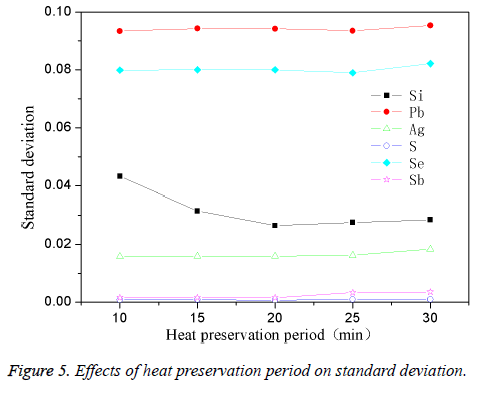
The high-purity copper was divided into several parts and put it into the intermediate frequency induction furnace with charcoal covered. Add in planned quantity of other element when the reaction temperature was 1200°C, stirring for 1min. The effect of different stirring time on standard deviation was shown in Figure 5. From Figure 5, it was clear that the standard deviation for most of the elements was larger when holding time was 10 min, especially evident for Pb, Se. Then standard deviation for most of the elements gradually tent to be smaller after extending the holding time and changed to be stable when the holding time was 25 min. In fact, appropriate holding time was very essential during the process of standard samples preparation. When holding time was too short, the impurity elements couldn’t be mixed evenly with the copper melt, so the standard deviation was higher; but lengthening holding time leads properly to 25 min, the impurities could mix evenly with the copper melt, so the standard deviation can gradually change to be stable. Thus the optimum holding time was 25 min.
The determination of the quantity of argon gas
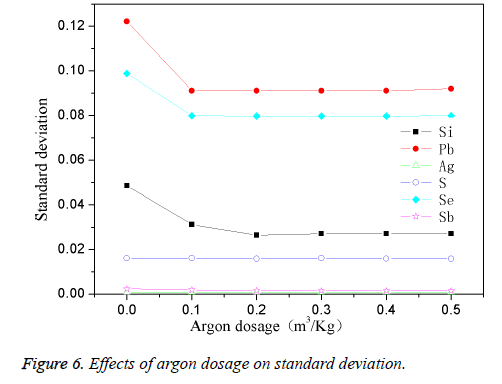
The high-purity copper was divided into several parts and put it into the intermediate frequency induction furnace with charcoal covered. Add in planned quantity of other element when the reaction temperature was 1200°C, stirring for 1 min, then preserved for 25 min. Changing the quantity of passed argon gas, the effects are shown in Figure 6. Figure 6 showed that when the system did not pass into the argon gas, the standard deviation for the maximum added elements reach the maximum; when argon fluxed into the increases, the standard deviation decreases, and gradually tend to stable when the volume of fluxed argon get to 0.4 m3/kg. This was because that argon gas can cut off the melt from the air, and prevent oxygen passing into the copper melt and impurity element from oxidation so as to improve the purity of the standard sample and precision, reduce the standard deviation analysis. When the quantity of argon gas reached the saturated value of 0.4 m3/kg, the standard deviation showed to stable. This experiment determined the suitable argon flux into the volume was 0.4 m3/kg.
Characterize of the standard samples
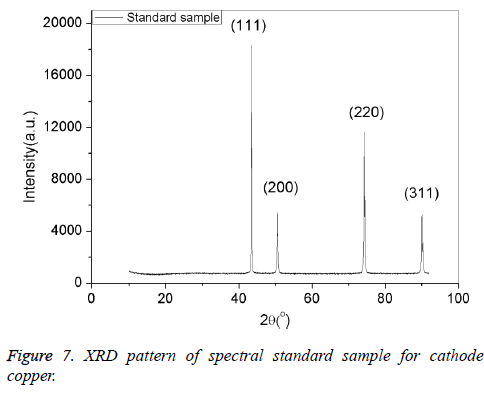
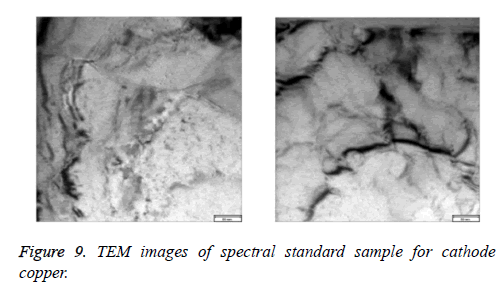
We can come to the conclusion that under the condition of casting temperature 1200°C, mixing speed 120 rpm, mixing time 1 min, holding time 25 min, and the dosage of argon gas 0.4 m3/kg , we can get the optimum spectral cathode copper standard samples. The samples were characterized by X-ray diffraction (XRD), Scanning electronic microscope (SEM), and transmission electron microscopy (TEM). Results were shown in Figures 7-9. From Figures 7-9, it can be seen that the standard samples prepared under the optimum condition are purity copper with compact structure and intensely diffraction peak compared with the standard card (PDF04-0836).
Constant Analysis of Standard Samples and Data Processing
Constant analysis
The standard samples prepared in the experiments were tested by eight authority lab using national analysis method, each experiment were conducted by at least two experienced analyst and more than four analyst results had been obtained.
Data processing
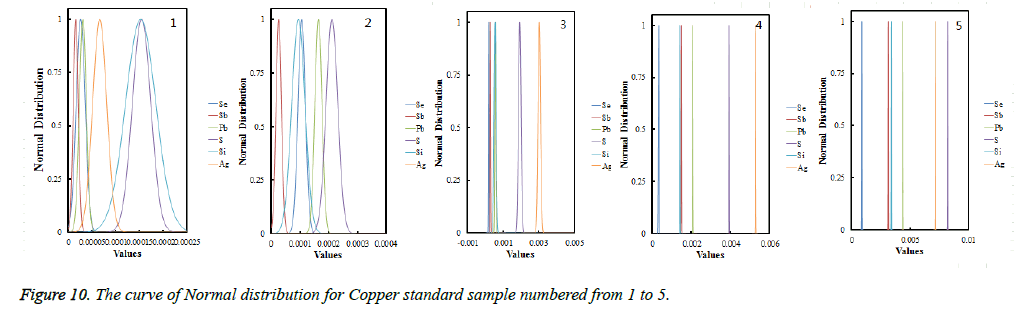
Each average values had been proved to obey the normal distribution using the Shapiro-Wilkes method [25], figures were shown in Figure 10; validating the results from collaborating laboratories using Dixon inspection and tradeoffs, all data had passed the Dixon inspection as both high-end statistics and low-end statistical value were smaller than the critical value; Cochran tests shows the results were at the same accuracy level.
Determination of the standard value
From the curve of normal distribution showing in Figure 10, we could see that the curve for Se, Sb, Pb, S, Si, Ag were completely symmetric about the vertical, about the standard value, with a steepen skew. And the curve graded increase with the increase of the content of copper standard samples from No. 1 to No. 5. Which showed that the smaller the Standard deviation will be, the more accurate the fixed value will be [26], so we could determine the standard value upon confirmation of fixed value data obeying the normal distribution or approximately obey the normal distribution and the abnormal value inspection and precision inspection test after remove outliers, the results were shown in Table 1.
| No | Data name | Se | Te | Bi | Cr | Mn | Sb |
|---|---|---|---|---|---|---|---|
| 1 | Standard values (%) | 0.00002 | 0.00003 | 0.00002 | 0.00002 | 0.00003 | 0.00002 |
| Standard deviation (%) | 0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.00001 | 0.00001 | |
| 2 | Standard values (%) | 0.00011 | 0.00007 | 0.00004 | 0.00003 | 0.00005 | 0.00003 |
| Standard deviation (%) | 0.00002 | 0.00002 | 0.00002 | 0.00001 | 0.00002 | 0.00001 | |
| 3 | Standard values (%) | 0.00019 | 0.00015 | 0.00014 | 0.00046 | 0.00042 | 0.00028 |
| Standard deviation (%) | 0.00002 | 0.00003 | 0.00002 | 0.00002 | 0.00002 | 0.00003 | |
| 4 | Standard values (%) | 0.00034 | 0.00041 | 0.00059 | 0.0012 | 0.0011 | 0.0015 |
| Standard deviation (%) | 0.00002 | 0.00003 | 0.00004 | 0.0001 | 0.0001 | 0.0001 | |
| 5 | Standard values (%) | 0.00087 | 0.0011 | 0.0012 | 0.0032 | 0.0027 | 0.0031 |
| Standard deviation (%) | 0.00006 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| No. | Data name | Cd | As | P | Pb | S | Sn |
| 1 | Standard values (%) | 0.00001 | 0.00001 | 0.00005 | 0.00003 | 0.00015 | 0.00001 |
| Standard deviation (%) | 0.00001 | 0.00001 | 0.00002 | 0.00002 | 0.00004 | 0.00001 | |
| 2 | Standard values (%) | 0.00013 | 0.00014 | 0.00015 | 0.00016 | 0.00021 | 0.00009 |
| Standard deviation (%) | 0.00001 | 0.00002 | 0.00004 | 0.00003 | 0.00004 | 0.00002 | |
| 3 | Standard values (%) | 0.00046 | 0.00057 | 0.00085 | 0.00055 | 0.0019 | 0.00055 |
| Standard deviation (%) | 0.00002 | 0.00003 | 0.00004 | 0.00003 | 0.0001 | 0.00004 | |
| 4 | Standard values (%) | 0.0012 | 0.0018 | 0.0026 | 0.0021 | 0.0039 | 0.0012 |
| Standard deviation (%) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | |
| 5 | Standard values (%) | 0.003 | 0.0038 | 0.0056 | 0.0044 | 0.0082 | 0.0026 |
| Standard deviation (%) | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0002 | 0.0001 | |
| No. | Data name | Ni | Fe | Si | Zn | Co | Ag |
| 1 | Standard values (%) | 0.00009 | 0.00016 | 0.00015 | 0.00004 | 0.00001 | 0.00007 |
| Standard deviation (%) | 0.00002 | 0.00003 | 0.00002 | 0.00002 | 0.00001 | 0.00002 | |
| 2 | Standard values (%) | 0.00021 | 0.00014 | 0.00009 | 0.00014 | 0.00013 | 0.0011 |
| Standard deviation (%) | 0.00003 | 0.00002 | 0.00003 | 0.00003 | 0.00001 | 0.0001 | |
| 3 | Standard values (%) | 0.00061 | 0.00066 | 0.00054 | 0.00093 | 0.00056 | 0.003 |
| Standard deviation (%) | 0.00003 | 0.00004 | 0.00004 | 0.00004 | 0.00003 | 0.0002 | |
| 4 | Standard values (%) | 0.0023 | 0.0024 | 0.0014 | 0.0026 | 0.0018 | 0.0053 |
| Standard deviation (%) | 0.0001 | 0.0001 | 0.0001 | 0.0002 | 0.0002 | 0.0002 | |
| 5 | Standard values (%) | 0.006 | 0.0054 | 0.0034 | 0.0052 | 0.0035 | 0.0072 |
| Standard deviation (%) | 0.0001 | 0.0002 | 0.0002 | 0.0003 | 0.0002 | 0.0002 |
Table 1. Standard value and deviation of standard sample.
Inspection of the working curve
The working curve was made at the optimum experimental condition, using the average intensity value and standard value of the tested element, which showed a good linear with the correlation coefficients more than 0.99.
Conclusion
Spectral cathode copper standard samples were prepared in the intermediate frequency induction furnace by adding necessary additives into 5N-level cathode copper. After batch experiments, we can get the optimized condition of preparation process were as follows: the casting temperature was 1200°C, the stirring rate was 120 rpm, the stirring time was 1 min, the heat preservation period was 25 min and argon dosage was 0.4 m3/kg. The design of the standard samples meet the requirements of cathode copper casting technology, the process obey the course of “technical specification for standard samples used in the analysis of the non-ferrous metal product analysis” (YS/T409). The spectral standard sample for cathode copper obtained under these conditions had a preferred orientation on the (220) plane due to its compact and uniform structure, stability, accuracy, and reliability.
References
- Hoseyin T. Adhesive wear resistance of Cu-Sn-Zn-Pb bronze with additions of Fe, Mn andPEJI. Materials Lett 2005; 59: 463-469.
- Aksoy M, Kuzucu V, Turhan H. A note on the effect of phosphorus on the microstructure and mechanical properties of lead- tin bronze. J Materials Process Technol 2002; 124: 113-119.
- Cura D’Ars de JF, de Freitas VCL, De Bellis VM. Surface characterization of a corroded bronze-leaded alloy in a salt spray cabinet J. Appl Sci 2007; 253: 7104-7107.
- Hurhan T, Aksoy M, Kuzucu V. The effect of man-ganese on the microstructure and mechanical properties of leaded-tin bronze. J Materials Process Technol 2001; 114: 207-211.
- Chiavari C, Colledan A, Frignani A. Corrosion evaluation of traditional and new bronzes for artistic castings. Materials Chem Physics 2006; 95: 252-259.
- Braun TB, Rawling JR, Richards KJ. Factors affecting the quality of electrorefining cathode copper. In: Yannopoulos, J.C., Agrwal, J.C. (Eds.), Extractive Metallurgy of Copper, vol. I. The Metallurgical Society, Inc., New York, 1976; 511-524.
- Davenport WG, King M, Schlesinger M. Extractive Metallurgy of Copper (Fourth ed.). Pergamon Press, New York, 2008; 270-277.
- Juhasz I, Constantin I, Hotea VP. Researches on the electrolyte purification and the useful elements recovery in the copper electrolytic refining process. Rev Roum Chim 2008; 53: 369-377.
- Xiao FX, Mao JW, Cao D. The role of trivalent arsenic in removal of antimony and bismuth impurities from copper electrolytes. Hydrometallurgy 2012.
- Caldas LFS, de Paula CER, Brum DM. Application of a four-variables Doehlert design for the multivariate optimization of copper determination in petroleum-derived insulating oils by GFAAS employing the dilute-and-shot approach. Fuel 2013; 105: 503-511.
- Li YC, Jiang SJ. Determination of Cu, Zn, Cd and Pb in fish samples by slurry sampling electrothermal vaporization inductively coupled plasma mass spectrometry. Analytica Chimica Acta 1998; 359: 205-212.
- Zawisza B, Sitko R. Determination of Te, Bi, Ni, Sb and Au by X-ray fluorescence spectrometry following electroenrichment on a copper cathode. Spectrochimica Acta Part B 2007; 62: 1147-1152.
- Piech R, Kubiak WW. Electrochimica Acta 2007; 53: 584-589.
- Raposo JL Jr, de Oliveira AP, Jones BT, Gomes Neto JA. Internal standardization combined with dilute-and-shoot preparation of distilled alcoholic beverages for Cu determination by high-resolution continuum source flame atomic absorption spectrometry. Talanta 2012; 92: 53-57.
- Packer AP, Gervasio APG, Miranda CES. On-line electrolytic dissolution for lead determination in high-purity copper by isotope dilution inductively coupled plasma mass spectrometry. Analytica Chimica Acta 2003; 485: 145-153.
- Davenport WG, King M, Schlesinger M. Extractive Metallurgy of Copper (Fourth ed.). Pergamon Press, New York, 2002: 270-277.
- Kamath BP, Mitra AK, Radhakrishnan S. Electrolyte impurity control at chinchpada refinery of sterlite industries (India) limited. Copper Electrorefining and Electrowinning 2003.
- Xiao F, Mao J, Cao D. The role of trivalent arsenic in removal of antimony and bismuth impurities from copper electrolytes [J]. Hydrometallurgy 2012; 8: 76-80.
- Xiao FX, Mao JW, Cao D. Formation of antimonate in co-precipitation reaction of As, Sb and Bi in copper electrolytes[J]. Minerals Eng 2012; 35: 9-15.
- Xiao FX, Cao D, Mao JW. Role of trivalent antimony in the removal of As, Sb, and Bi impurities from copper electrolytes[J]. Int J Minerals Metallur Mater 2013; 20: 9-16.
- Xiao FX, Cao D, Mao JW. Role of Sb(V) in removal of As, Sb and Bi impurities from copper electrolyte[J]. Transact Nonferrous Metals Soc China 2014; 24: 271-278.
- Chinese National Standard of GB/T 15000 for working guideline of standard sample.
- The standard sample specification of YS/T 409-1998 for analysis of non-ferrous metal product.
- Hao Q. Standard substance and its application technology, Beijing: China Standards Press, 2003.
- Ming BSY. Nonferrous Metals Industry standard assembly - heavy metal and its alloy products, China Standards Press, Beijing, 2000.
- Rules of rounding off of numerical values and the representation and judgments of limit value, Standardization Administration of China, GB/T 8170-2008.