- Biomedical Research (2015) Volume 26, Issue 3
Study on the chemical constituents of Momordica charantia L. leaves and method for their quantitative determination.
Wen Li1,2, Zhaozhou Lin1,2, Chengjie Yang2, Yubo Wang2, Yanjiang Qiao1*1College of Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100102, China;
2School of Pharmacy, Lanzhou University, Lanzhou 730000, China.
- *Corresponding Author:
- Yanjiang Qiao
College of Chinese Medicine
Beijing University of Chinese Medicine
No.6 Wangjing Central Ring Road
haoyang Beijing 100102, China
Accepted date: December 05 2014
Abstract
Momordica charantia L. is a herbaceous climbing plant in the genus Momordica of family Cucurbitaceae. It is to study the chemical constituents of Momordica charantia L. leaves and establish the method for determination of their total saponin content. Momordica charantia L. leaves are isolated and purified by silica gel and Sephadex LH-20 column chromatographies, and the resulting compounds are structurally elucidated by NMR spectroscopy. Total saponins in Momordica charantia L. leaf extract are quantitatively determined by spectrophotometry with Momordicin I as the standard substance. Five compounds are isolated from the 80% ethanol extract of Momordica charantia L. leaves, which are identified as Momordicin I (1), Momordicin IV (2), Aglycone of Momordicoside ?(3), Aglycone of Momordicoside L (4) and Karavilagenin D (5), respectively. Total saponins have a good linear relationship with the absorbance within a range of 0.005~0.025 μg, recovery is 100.27%, and RSD= 1.20% (n= 6). The isolated compounds are all triterpenoids. The present quantitative determination method is simple, fast and accurate, which can be used as the method for determination of triterpenoid content in Momordica charantia L. leaves.
Keywords
Momordica charantia L. leaf; chemical constituent; quantitative determination; 1H-NMR; 13C-NMR; hypoglycemic activity
Introduction
Momordica charantia L., also known as Jinlizhi, Laiputao, Laigua, Lianggua, etc., is a herbaceous climbing plant in the genus Momordica of family Cucurbitaceae, which is widely distributed in tropical, subtropical and temperate regions. Momordica charantia L. is bitter in taste and cold in nature, which is used in the treatment of fever with thirst, heat stroke, dysentery, dye redness & pain, carbuncles, erysipelas, malignant sores, etc. [1]; it is a common folk food and medicine. In recent years, research on Momordica charantia L. has been concentrated on fruits and seeds, and a variety of chemical constituents have been isolated and purified from fruits and seeds of Momordica charantia L. [2-4]. Modern medical studies have found that Momordica charantia L. possesses hypoglycemic, anti-tumor and immunity enhancing actions [5-7]. Research has reported that the diversity of hypoglycemic constituents in Momordica charantia L. indicates the hypoglycemic effect of Momordica charantia L. may be exerted synergistically by a variety of substances through multiple pathways. To further clarify the hypoglycemic mechanism of Momordica charantia L. and efficiently utilize Momordica charantia L. leaf resources, in-depth study on hypoglycemic substance basis of Momordica charantia L. is necessary. Therefore, this paper studies the 80% ethanol extract of Momordica charantia L. leaves, isolates five kinds of triterpenoids from it, and determines the content of these triterpenoids in the Momordica charantia L. leaf extract.
Instruments and Materials
WRS-3 melting point apparatus (Shanghai Precision & Scientific Instruments Co., Ltd.); L5 UV/Vis spectrometer (Shanghai INESA Analytical Instrument Co., Ltd.); FTIR- 650 Fourier transform infrared spectrometer (Tianjin Gangdong Sci & Tech Development Co., Ltd.); INOVA- 400 NMR spectrometer (Varian, USA); DECAX-30000 LCQ Deca XP (Thermo Finnigan). BC66 ultrasonic cleaner (Nanjing Kepurui Ultrasonic Equipment Co., Ltd.); column chromatography silica gel, product of Qingdao Haiyang Chemical Plant; silica gel GF254 TLC plate, product of Yantai Institute of Chemical Industry; Sephadex LH-20 column chromatography material, product of Pharmacia; HPLC grade methanol, product of Jiangsu Hanbon Sci & Tech Co., Ltd; other reagents were all chemically or analytically pure.
Momordica charantia L. leaf sample was purchased from Hebei Anguo medicine market, which was identified by Professor Fu Qingfang from Chengdu University of TCM in Sichuan as Cucurbitaceae plant Momordica charantia L. Momordicin I (self-prepared, for determination of total saponin content, purity> 98%);
Study of the chemical constituents
Extraction and isolation
6 kg of dried Momordica charantia L. leaves were pulverized, and soaked in 80% ethanol at room temperature for one week; the extracts were then combined, and concentrated under reduced pressure to obtain the total extract. The total extract was suspended in appropriate amount of water, and extracted successively with equivalent amounts of petroleum ether, ethyl acetate and n-butanol; the extracts were then combined, and concentrated under reduced pressure to give petroleum ether fraction, n-butanol fraction and water fraction, respectively. The n-butanol fraction was repeatedly subjected to silica gel column chromatography (chloroform-methanol system), and purified by Sephadex LH-20 column (chloroform-methanol, methanol-water systems) as well as preparative HPLC; finally, a total of five compounds were isolated.
Structure identification
Compound 1: white powder, ESI-MS m/z: 495 [M+Na]+ 。IR υmax (cm−l) : 3452, 2949, 1645, 1553, 1451, 975。 1H-NMR (400 MHz, CD3OD) δ: 0.80, 0.94, 1.09, 1.26 (各 3H, s, H-18, 28, 29, 30), 1.08 (3H, d, J = 6.4 Hz, H-21), 1.66 (3H, s, H-26), 1.72 (3H, s, H-27), 1.97 (1H, m, H-20), 2.46 (1H, m, H-8), 2.60 (1H, m, H- 10), 3.63 (1H, brs, H-3), 4.06 (1H, d, J = 5.2 Hz, H-7), 4.47 (1H, m, H-23), 5.18 (1H, d, J = 8.8 Hz, H-24), 5.90 (1H, d, J = 4.0 Hz, H-6), 9.80 (1H, s, H-19); 13C-NMR (100 MHz, CD3OD) δ: 24.1 (C-1), 32.1 (C-2), 78.2 (C-3), 42.5 (C-4), 146.3(C-5), 123.2 (C-6), 67.4 (C-7), 49.8 (C- 8), 53.2 (C-9), 38.2 (C-10), 24.9 (C-11), 30.1 (C-12), 46.9 (C-13), 48.2 (C-14), 36.1 (C-15), 29.4 (C-16), 52.1 (C- 17), 15.6 (C-18), 209.9 (C-19), 32.9 (C-20), 19.7 (C-21), 45.7 (C-22), 68.2 (C-23), 135.4 (C-24), 131.5 (C-25), 18.9 (C-26), 26.7 (C-27), 26.2 (C-28), 28.4 (C-29), 18.5 (C-30). The above data was consistent with the reported literature [8], so compound 1 was identified as Momordicin I.
Compound 2: white powder, ESI-MS m/z: 657 [M+Na]+ 。IR υmax(cm−l): 3447, 2949, 1651, 1553, 1457, 967. 1H-NMR (400 MHz, pyridine-d5) δ: 0.88, 0.91, 1.14, 1.49 (each 3H, s, H-18, 28, 29, 30), 1.21 (3H, d, J = 6.4 Hz, H-21), 1.75 (3H, s, H-26), 1.83 (3H, s, H- 27), 2.12 (1H, m, H-20), 2.41 (1H, m, H-8), 2.81 (1H, m, H-10), 3.85 (1H, brs, H-3), 4.31 (1H, d, J = 5.2 Hz, H-7), 5.01 (1H, d, J = 7.8 Hz, H-1′), 5.61 (1H, d, J = 7.8 Hz, H- 24), 6.31 (1H, d, J = 4.4 Hz, H-6), 10.67 (1H, s, H-19); 13C-NMR (100 MHz, pyridine-d5) δ: 21.0 (C-1), 28.1 (C- 2), 75.1 (C-3), 41.2 (C-4), 144.6 (C-5), 124.9 (C-6), 65.8 (C-7), 50.3 (C-8), 50.2 (C-9), 36.9 (C-10), 22.0 (C-11), 29.9 (C-12), 46.3 (C-13), 48.1 (C-14), 35.0 (C-15), 27.7 (C-16), 51.6 (C-17), 14.7 (C-18), 207.9 (C-19), 32.0 (C- 20), 19.1 (C-21), 43.5 (C-22), 74.8 (C-23), 129.6 (C-24), 133.1 (C-25), 18.3 (C-26), 26.8 (C-27), 25.7 (C-28), 27.6 (C-29), 18.3 (C-30), 104.8 (C-1′), 75.5 (C-2′), 78.6 (C-3′), 71.6 (C-4′), 78.5 (C-5′), 63.2 (C-6′). The above data was consistent with the reported literature [9], so compound 2 was identified as Momordicin IV.
Compound 3: colorless flaky crystal (methanol), Liebermann- Burchard reaction was purple, and Molish reaction was negative. UV λ203 nm。 1H-NMR (400 MHz, CDCl3) δ: 6.07 (H, dd, J = 9.8, l.8 Hz), 5.68 (H, dd, J = 9.8, 3.6 Hz), 5.62 (2H, m), 4.08 (H, brd); 13C-NMR (100 MHz, CDCl3) δ: 18.8 (C-1), 27.8 (C-2), 75.2 (C-3), 39.8 (C-4), 87.7 (C-5), 132.1 (C-6), 133.1 (C-7), 52.5 (C-8), 44.9 (C-9), 39.5 (C-10), 24.9 (C-11), 31.5 (C-12), 45.9 (C-13), 48.9 (C-14), 35.0 (C-15), 29.9 (C-16), 50.1 (C- 17), 14.6 (C-18), 79.6 (C-19), 36.2 (C-20), 17.6 (C-21), 37.2 (C-22), 126.8 (C-23), 140.2 (C-24), 71.2 (C-25), 30.9 (C-26), 28.0 (C-27), 21.2 (C-28), 24.6 (C-29), 20.1 (C-30). 以上数据与文献报道一致[10],故鉴定化合物3 为Aglycone of MomordicosideⅠ. The above data was consistent with the reported literature [10], so compound 3 was identified as Aglycone of MomordicosideⅠ.
Compound 4: colorless flaky crystal (acetone), Liebermann- Burchard reaction was purple, and Molish reaction was negative. 1H-NMR (400 MHz, CDCl3) δ: 9.75 (H, s), 5.92 (H, brd, J = 4.6 Hz), 5.58 (2H, m), 0.76, 0.90, 0.92, 1.08, 1.26, 1.32, 1.34 (CH3×7), 3.58 (H, brs), 3.99 (H, brd, J=5.2Hz); 13C-NMR (100 MHz, CDCl3) δ: 21.4 (C- 1), 29.5 (C-2), 77.0 (C-3), 42.6 (C-4), 146.9 (C-5), 124.8 (C-6), 66.6 (C-7), 50.1 (C-8), 50.1 (C-9), 36.7 (C-10), 25.1 (C-11), 28.6 (C-12), 45.3 (C-13), 47.2 (C-14), 34.9 (C-15), 28.1 (C-16), 50.2 (C-17), 15.2 (C-18), 208.1 (C- 19), 36.2 (C-20), 18.9 (C-21), 39.2 (C-22), 125.3 (C-23), 140.4 (C-24), 70.5 (C-25), 30.1 (C-26), 29.0 (C-27), 25.2 (C-28), 27.9 (C-29), 18.5 (C-30). The above data was consistent with the reported literature [10], so compound 4 was identified as Aglycone of Momordicoside L.
Compound 5: white powder (acetone). 1H-NMR (C5D5N, 400 MHz) d: 0.85, 0.92, 0.95, 1.27 (3HÃ4, all s, H-29, H- 18, H-28, H-30 ), 5.67 (2H, m, H-23, 24 ); 13C-NMR (C5D5N, 100 MHz) d: 18.9 (C-1), 27.7 (C-2), 73.8 (C-3), 37.9 (C-4), 84.9 (C-5), 133.1 (C-6), 133.2 (C-7), 45.2 (C- 8), 51.6 (C-9), 40.9 (C-10), 22.6 (C-11), 31.1 (C-12), 45.9 (C-13), 48.0 (C-14), 34.9 (C-15), 27.3 (C-16), 50.1 (C-17), 14.7 (C-18), 182.2 (C-19), 36.7 (C-20), 19.8 (C-21), 39.6 (C-22), 123.8 (C-23), 142.9 (C-24), 69.9 (C-25), 30.8 (C- 26), 31.6 (C-27); 19.9 (C-28), 26.0 (C-29), 21.7 (C-30). The above data was consistent with the reported literature [11], so compound 5 was identified as Karavilagenin D.
Study of quantitative determination
Preparation of solutions
Preparation of reference solution: 25 mg of Momordicin I reference substance, which was dried to constant weight at 105°C, was accurately weighed, placed in a 50 mL volumetric flask, dissolved in methanol, diluted to the mark, and shaken well. 5.0 mL of the resulting solution was precisely drawn, placed in a 10 mL volumetric flask, diluted to the mark with methanol and shaken well to prepare a 0.25mg/mL reference solution.
Preparation of test solution: 100 mg of n-butanol fraction extract, which was dried to constant weight at 105°C, was accurately weighed, placed in a 50 mL volumetric flask, dissolved in methanol, diluted to the mark, and shaken well. 5.0 mL of the resulting solution was precisely drawn, placed in a 10 mL volumetric flask, diluted to the mark with methanol and shaken well to prepare the test solution.
Quantitative determination method
50 µl of test solution was precisely drawn, placed into corresponding colorimetric tubes, and heated in water bath to evaporate methanol, then taken out. After drying at 60°C, each colorimetric tube was added with 0.2 mL of 5% vanillin-glacial acetic acid solution, as well as 0.8 mL of perchloric acid, shaken well, and heated in water bath at 60°C for 15 minutes, then removed and cooled immediately. Afterwards, each colorimetric tube was added with 4 mL of glacial acetic acid solution, shaken well, and measured at a 573 nm wavelength.
Results
Establishment of quantitative index and method for determination of total saponin content in Momordica charantia L. leaves
The extract in this paper is from the leaves of Momordica charantia L., literatures have reported that Momordica charantia L. leaves mainly contain sterols, saponins and their derivatives, all of which have the steroidal nucleus structure. This in combination with the fact that relatively many sterols and saponins have been isolated, total saponin content in Momordica charantia L. leaves was determined by spectrophotometry with Momordicin I as the reference substance.
Investigation of test solution preparation method
Momordicin I was completely soluble in methanol, and had similar UV absorbance to Momordica charantia L. leaf extract, so it could be completely dissolved by ultrasonication with methanol as the test solution.
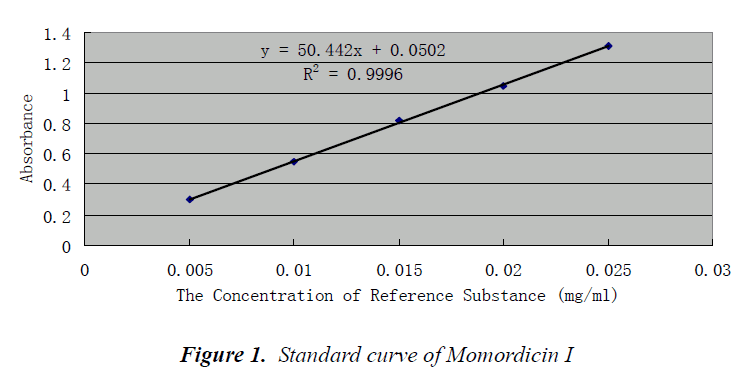
Investigation of linear range
0, 20, 40, 60, 80 and 100 µL of reference solutions were precisely drawn into corresponding colorimetric tubes, and heated in water bath to evaporate methanol, then taken out. After drying at 60°C, each colorimetric tube was added with 0.2 mL of 5% vanillin-glacial acetic acid solution, as well as 0.8 mL of perchloric acid, shaken well, and heated in water bath at 60°C for 15 minutes, then removed and cooled immediately. Afterwards, each colorimetric tube was added with 4 mL of glacial acetic acid solution, shaken well, and measured at a 573 nm wavelength. The results are shown in Table 1.
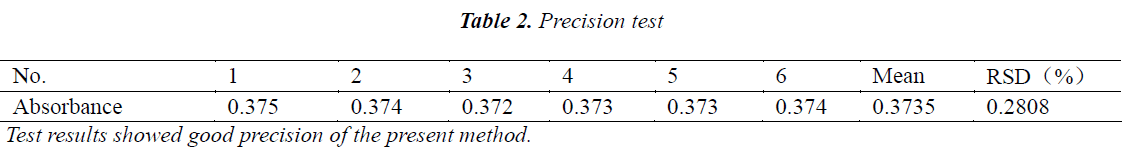
Precision test
50 µL of aliquots of the same test solution were precisely drawn, and tested continuously six times, the results are shown in Table 2.
Standard curve was plotted with the concentration of reference substance as abscissa and the absorbance integral as ordinate, and the regression equation was obtained as follows: Y = 50.442X + 0.0502, R = 0.9996. Reference substance had a good linearity within 0.005~0.025 mg/mL
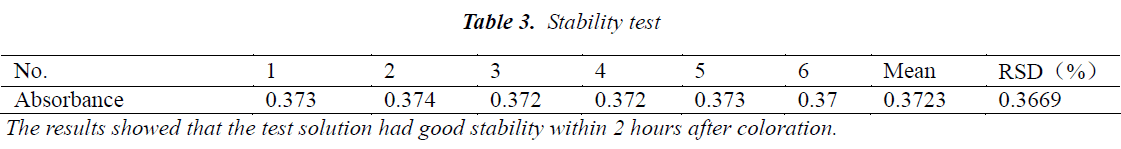
Stability test
50 µL of aliquots of the same test solution were precisely drawn, and tested for content six times 0, 30, 60, 90, 120 and 150 minutes after preparation, respectively, followed by calculation of mean and RSD. The results are shown in Table 3.
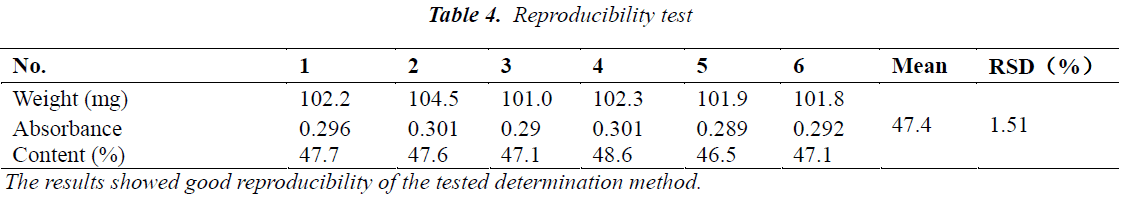
Reproducibility test
Six aliquots of n-butanol fraction samples of Momordica charantia L. leaf extract were taken, and operated as per the method under "Quantitative determination method" to determine total saponin content. The results are shown in Table 4.
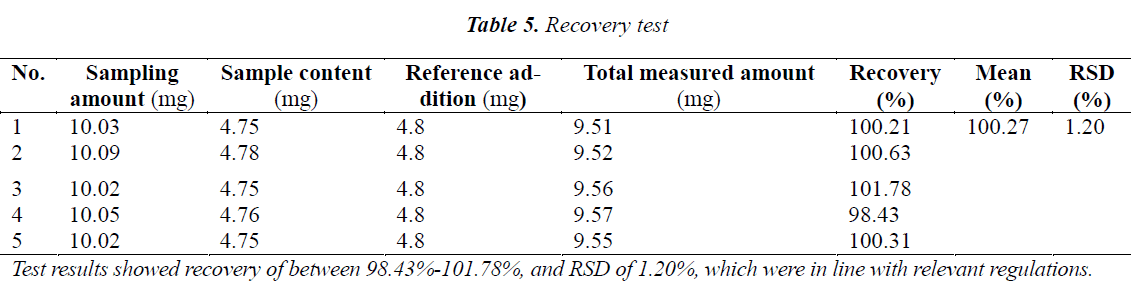
Recovery test
10 mg of n-butanol fraction of Momordica charantia L. leaf extract with a known content (47.4%) was accurately weighed, added precisely with 4 mL of Momordicin I reference solution (1.2 mg/mL), and operated as per the method under "Quantitative determination method". The results are shown in Table 5.
Discussion
Diabetes is a chronic metabolic disease due to metabolic disturbance, and insulin production and action disorders [12]. With the growth in the number of diabetic patients, diabetes has become the third biggest disease category threatening human health after cardiovascular diseases and tumor, and one of the highly prevalent diseases in modern society; not only its group incidence tends to increase, its age of onset also tends younger. How to effectively lower blood sugar levels is increasingly becoming the focus of preventive medical professionals. The search for active hypoglycemic constituents from natural medicines, especially herbal medicines is an important approach.
In Asia, Momordica charantia L. is used as a medicine and food. Modern research has found that Momordica charantia L. has a good hypoglycemic activity. Chemical constituents isolated from Momordica charantia L. have manifested insulin- like effect or can promote the release of insulin, demonstrating the insulin-like properties of Momordica charantia L. [13]. Research has confirmed that the hypoglycemic effect of Momordica charantia L. is achieved through the following several ways: 1. reducing hepatic gluconeogenesis, increasing hepatic glycogen synthesis; 2. increasing glucose oxidation around red blood cells and fat cells [14].
To further utilize Momordica charantia L. resources, and make full use of Momordica charantia L. leaves, we conducted in-depth study on its chemical constituents and contents. Studies have reported that major constituents of Momordica charantia L. are triterpenoids [11,15-17]; in this study, we isolated five triterpenoids with similar nuclei from Momordica charantia L. leaves, and confirmed that triterpenoids are not only the main constituents of Momordica charantia L., but also the main constituents of Momordica charantia L. leaves. We selected Momordicin I, a constituent with relatively high content, as the quantitative determination index to study its content determination method. At present, there are a lot of hypoglycemic drugs that contain Momordica charantia L. constituents, such as Kugua Jiangtang Capsule, Kugua Huoyi Jiangtang Capsule and Momordicin Capsule. This category of drugs has high sales volume and popularity, whose quality control needs to be further improved. Therefore, we studied the method for determination of triterpenoids in Momordica charantia L. leaves; the method established enables simple, fast and accurate determination of triterpenoids in Momordica charantia L. leaves. To ensure safety and effectiveness of drugs, the method provides a basis for quality control of Momordica charantia L. containing drugs.
References
- Jiangsu New Medical College. Dictionary of Chinese Materia Medica. Shanghai: Shanghai Scientific and Technical Publishers 1986: 123-124.
- Ma L, Yu AH, Sun LL, Gao W, Zhang MM, Su YL, Liu H, Ji TF, Li DZ. Two new Cucurbitane triterpenoids from the seeds of Momordica charantia. Journal of Asian Natural Products Research 2014; 16: 476-482.
- Zeng K, He YN, Yang D, Cao JQ, Xia XC, Zhang SJ, Bi XL, Zhao YQ. New compounds from acid hydrolyzed products of the fruits of Momordica charantia L. and their inhibitory activity against protein tyrosine phosphatas 1B. European journal of medicinal chemistry 2014; 81: 176- 180.
- Li QY, Chen HB, Liu ZM, Wang B, Zhao YY. Cucurbitane triterpenoids from Momordica charantia. Magnetic Resonance in Chemistry 2007; 45: 451-456.
- Nkambo W, Anyama NG, Onegi B. In vivo hypoglycemic effect of methanolic fruit extract of Momordica charantiaL.. African Health Sciences 2013; 13: 933-939.
- Hsiao PC, Liaw CC, Hwang SY, Cheng HL, Zhang LJ, Shen CC, Hsu FL, Kuo YH. Antiproliferative and Hypoglycemic Cucurbitane-Type Glycosides from the Fruits of Momordica charantia. J Agric Food Chem 2013; 61: 2979-2986.
- Fredulin SE, Cristina HFA, Castro PHC, Paula BRA, Aparecida CM, LuzÃaFE. Immunomodulatory Effects of Poly (ethylene glycol) Microspheres Adsorbed with Nanofractionsof Momordica charantiaL. on Diabetic Human Blood Phagocytes. Science of Advanced Materials 2011; 3: 687-694.
- Ma J, Whittaker P, Keller AC, Mazzola EP, Pawar RS, White KD, Callahan JH, Kennelly EJ, Krynitsky AJ, Rader JI. Cucurbitane-type triterpenoids from Momordica charantia.Planta Med 2010; 76: 1758-1761.
- Kashiwagi T, Mekuria D B, Dekebo A S, Sato K, TebayashiS, Kim CS. A new oviposition deterrent to the leafminer, Liriomyzatrifolii: cucurbitaneglucoside from Momordica charantia. Z Naturforsch C 2007; 62: 603- 607.
- Pan H, Zhao YQ. Chemical constituents in immature fruits of Momordica charantia. Chinese Traditional and Herbal Drugs 2007; 38: 9-11.
- Matsuda H, Nakamura S, Murakami T. Structures of new cucurbitane-type triterpenes and glycosides, karavilageninsD and E, and karavilosides VI, VI1, VIII, IX, X and XI, from the fruit of Momordica CharantiaL.. Heterocycles2007; 71: 331-341.
- Teramoto T, Sasaki J, Ishibashi S, Birou S, Daida H, DohiS, Egusa G, Hiro T, Hirobe K, Iida M, Kihara S, Kinoshita M, Maruyama C, Ohta T, Okamura T, Yamashita S, Yokode M, Yokote K. Diabetes mellitus. J Atheroscler Thromb 2014; 21: 93-98.
- Ng TB, Wong CM, Li WW, Yeung HW. Isolation and characterization of a galactose binding lectin with insulinm in etic activities from the seeds of the bitter gourd.Momordica charantia (family Cucurbita-ceae).Int J Pept Protein Res 1986; 28: 163-172.
- Shibib BA, Khan LA, Rahman R. Hypoglycaemic activity of Cocciniaindicaand Momordica charantiain diabetic rats: depression of the hepatic gluconeogenic enzymes glucose-6-phosphatase and fructose-1, 6-bisphosphatase and elevation of both liverand red-cell shuntenzyme glucose- 6-phosphate dehydrogenase. Biochem J 1993; 292(pt11): 267-270.
- Nakamura S, Toshiyuki M, Nakamura J. Structures of new cucurbitane-type triterpenes and glycosides, karavilageninsand karavilosides, from the dried fruit of Momordica charantia L. in Sri Lanka. Chem pharm bull 2006; 54: 1545-1550.
- Harinantenaina L, Tanaka M, Takaoka S, Oda M, MogamiO, Uchida M, Asakawa Y. Momordica charantia constituents and antidiabetic screening of the isolated major compounds. Chem pharm bull 2006; 54: 1017-1021.
- Tanm J, Yej M, Turner N. Antidiabetic activites of triterpenoidsisolated from bitter melong associated with activation of the AMPK pathway. Chemistry and biology 2008; 15: 263-273.