Research Article - Biomedical Research (2017) Volume 28, Issue 14
Study on the application of immune index such as CT enhanced cyclooxygenase 2, etc. in the diagnosis of lung cancer
Jiang Chang1, Xueying Zhang1, Jinping Zhang2, Fushan Chang3 and Xueying Zhang1*
1College of Information Engineering, Taiyuan University of Technology, Taiyuan , PR China
2College of Life Sciences, Hebei Agricultural University, Baoding, PR China
3Department of Physics and Electronic Engineering, Yuncheng University, Yuncheng, PR China
- *Corresponding Author:
- Xueying Zhang
College of Information Engineering
Taiyuan University of Technology,PR China
Accepted on June 07, 2017
Abstract
Background: Because of the angiogenic process of malignant tumours, there was significant difference between lung cancer blood supply and benign lesion. The Vascular Endothelial Growth Factor (VEGF) and Cyclooxygenase-2 (COX-2) is associated with the progression and poor prognosis of lung cancer. The aim of the study was to assess the correlation between the expression of COX-2, VEGF and Micro Vessel Density (MVD) and CT enhanced performance in lung cancer.
Methods: 25 cases of pathologically confirmed lung cancer and 35 cases of benign lung lesions were conducted by CT enhanced scanning, and the PV method was used for immunohistochemical analysis in the pathological specimen as well as the relationship between expression level of COX-2, VEGF, MVD and CT enhanced peak, histological type, clinical stage, lymph node metastasis and differentiation degree in lung cancer.
Results: Expression levels of COX-2, VEGF and MVD in the lung cancer group and control group; expression level of COX-2, VEGF and MVD is closely related to histological type, clinical stage, lymph node metastasis and CT enhanced peak in lung cancer, and has nothing to do with the degree of differentiation of lung cancer.
Conclusion: Our results showed that the expression of COX-2, VEGF and MVD in lung cancer is positively related to the peak value of CT enhancement, which indicated that CT enhancement can provide a non-invasive and non-invasive method for the diagnosis and evaluation of lung cancer.
Keywords
Lung cancer, Immunohistochemistry, Cyclooxygenase-2 (COX-2), Vascular endothelial growth factor (VEGF), Microvessel density (MVD).
Introduction
Tumour is an angiogenesis dependent disease [1]. It is reported that the expression of Vascular Endothelial Growth Factor (VEGF) and Cyclooxygenase 2 (COX-2) are related to the progression and poor prognosis of lung cancer [2,3]. Zang et al. suggested elevated serum levels of VEGF could predict a poor prognosis of platinum-based chemotherapy in non-small cell lung cancer [4]. The VEGF family played a significant role in the formation of blood vessels and lymphatic’s [5]. Recently, high expression of COX-2 had been confirmed in non-small cell lung cancer progression and was related to tumour invasion and metastasis [6]. Furthermore, COX-2 expression was significantly increased in nodal metastasis compared to primary cancers [7,8]. The angiogenesis quantification standard is the count of micro vessel density (MVD) [9].
Due to the process of angiogenesis in malignant tumour, there was significant difference between lung cancer blood supply and benign lesion [10]. And computed tomography (CT) could display tumour angiogenesis [11]. Based on the changes of tissue density after injection of contrast agent, this research conduct CT dynamic contrast-enhanced scan of 25 cases of lung cancer to obtain tumour CT enhanced peak value; and analysed the expression level of COX-2, VEGF, MVD in pathological specimens by immunohistochemical to study their relationship, in order to infer some malignant biological behaviour and prognosis from the perspective of CT, and to explore the clinical value of this method in the diagnosis of lung cancer.
Materials and Methods
Clinical data
From July 2004 to Feb 2006, 60 cases of patients, 25 cases of lung cancer, and 35 cases of the control group. An acid fast bacillus was found through surgical pathology and sputum culture or clinical confirmed. Among them, 42 were male and 18 were female with the age ranging from 22-74 y old. The staging was conducted according to the 1986 TNM staging method of the AJCC. The surgical doctor marked the focus in the operation, and embedded the entire lesion specimens by paraffin slice with slice direction consistent with CT fault as far as possible. CT images were compared to pathological sections to analyse the pathological basis of different enhancement features. The tumour enhancement patterns were observed by 3 experienced doctors under the condition of unknown pathological results.
CT examination
All the patients without preoperative radiotherapy and chemotherapy were instructed to hold the breath in calm inspiratory state before scanning. Before dynamic scanning, the pre scan was used to determine the best level of displaying lesions. SIEMENS Somatom Sensation 16 helical CT was used. Conventional unenhanced: 120 kV, 200 mA, scanning time 0.5 s, 512 × 512 matrix, FOV 30 cm. Lesions were chosen from thick layer with distance of 2 mm and the standard image to conduct reconstruction. The conditions of enhanced scanning were consistent with those of the unenhanced. Using 350 mgI/ml Omnipaque 90~100 ml and high pressure syringe at the flow rate of 3 ml/s through cubical vein of the contralateral lesion to do bolus injection. Scanning was begun 30 s after the injection, and then delays scanning in 1-7 min after injection. Lesions in the central level were chosen for measurement of CT value with the measuring range including 60% circular area of lesion’s short diameter. The parameters were recorded after dynamic enhanced scan: unenhanced CT value, enhanced peak (i.e. maximum enhanced CT value minus value of unenhanced). The average value of CT in 3 levels was seen as CT value of the lesion in this phase.
The immunohistochemical experiment data performed by PV method
The collection and processing of specimens. All the wax block specimens were from the Department of Pathology of the second hospital of Harbin Medical University and diagnosed by it. All the immunohistochemical reagents used in this experiment were purchased from Beijing Zhongshan Biotech Corp.
Immunohistochemical method
The preparation before dyeing: 1. Making washing agent PBS (Phosphate Buffer). 2. Preparing kit (includes reagent Amouse anti human VEGF monoclonal antibody; reagent Bmouse anti human cyclooxygenase-2 monoclonal antibody; reagent C-mouse anti endothelial monoclonal antibody; reagent D-Immuno-Bridge reagent: 3H2O2 deionized water, reagent 1: Polymer Helper (ready to use), reagent 2: Polyperoxi-daseanti- mouse IgG reagent (ready to use)).
Immunohistochemical staining: 1. De-waxing and hydrating tissue sections. 2. Citrate buffer used for antigen repair under high pressure. 3. 3H2O2 deionized water incubating for 10 min to block endogenous peroxidase. 4. Dripping first antibody at 4°C overnight in wet box and PBS washing, 5 min × 3 times. 5. Dropping agent 1, incubating at room temperature for 20 min, and PBS washing. 6. Dropping agent 2, incubating at room temperature for 30 min, and PBS washing. 7. Applying DAB solution to wash colour. 8. Rinsing with tap water, restaining, dehydrating, transparenting and mounting.
Immunohistochemical results: The total score of cell staining intensity and positive cell percentage was used to determine the expression of COX-2 and VEGF [12]. 5 high power field (400 times) were randomly selected for judgment: 1. No staining was recorded 0 point, 1 point of light yellow, pale brown 2 points and yellow-wish brown 3 points. 2. The positive rate of cell ≤ 5% was recorded 0 point, 5%~25% 1 point, 25%~50% 2 points, and >50% 3 points. By the sum of the above two, 0 point for the negative (-), 1~2 for weakly positive (1+), 3~4 moderately positive (2+), 5~6 strong positive (3+). Detection method of MVD was Weidner [13]. Low power lens (40 times) was first used to find out the three regions of the highest vascular density, and then the number of micro vessels were counted dyeing CD34 into pale brown under high power lens (200 times), and the result was expressed by the average number of vessels in 5 lens, 200 times of the vision.
Statistical processing
T-test, F-test and q-test were adopted, as well as linear correlation analysis and linear regression analysis. P<0.05 meant statistical significance. All the statistics were completed by SAS8.1 software.
Results
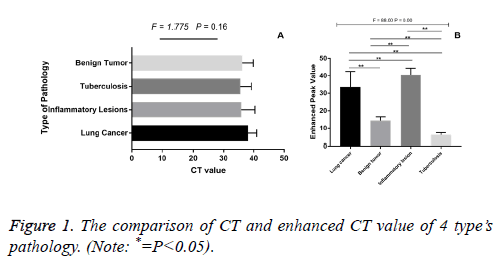
The unenhanced CT value among lung cancer, tuberculosis, inflammatory lesions and benign tumour had no significant difference (F=1.775, P>0.05, Figure 1A). However, the CT enhanced peak among four lesions of lung cancer, tuberculosis, inflammatory lesions, and benign tumour were statistically significant (F=88, P<0.01). The enhanced peak of lung cancer were higher than those of benign tumour and tuberculosis (P<0.01), but lower than those of inflammatory lesion (P<0.01, Figure 1B).
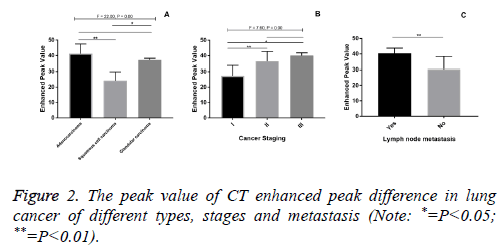
In lung cancer, CT enhanced peak values in adenocarcinoma group and adenosquamous carcinoma group were higher than that of squamous cell carcinoma group (P<0.05). CT enhanced peak values in stage II, stage III lung cancer were significantly higher than that in stage I (P<0.05). CT enhanced peak value of lung cancer with lymph node metastasis was significantly higher than that in non-metastasis group (P<0.01) (Figure 2).
The value of VEGF, COX-2, MVD in lung cancer group were higher than control group (t=2.31, P<0.05; t=2.75, P<0.05; t=2.29, P<0.05). Linear regression analysis was adopted to analyse the relationship of CT enhanced peak in lung cancer and expression values of the three indexes, and the equation was established as follows: VEGF (Y=1.362 × X+18.43, R=0.89, P<0.05); COX-2 (Y=2.82 × X+61.56, R=0.89, P<0.05); MVD (Y=2.042 × X+47.88, R=0.89, P<0.05). The results indicated that CT enhanced peak, VEGF, COX-2 and MVD expression values were positively correlated.
Discussion
Zhang et al. summed up the enhanced scanning results in 65 cases of non-calcified solitary pulmonary nodules: malignant tumour (41.9 ± 2.8) Hu, inflammatory lesions (43.6 ± 7.7) Hu, and the degree of enhancement in both was similar [14]. Ceng Qingsi et al. reported enhancement values of 3 cases were greater than 60 Hu in 7 cases of inflammatory nodules [15]. He believes that when enhancement value of the lesion is greater than 60 Hu, the possibility of inflammatory lesion is greater. In this study, the enhanced peak of inflammatory lesions was significantly higher than those of benign tumour and tuberculosis with no statistically significant difference between lung cancers. Therefore, if only enhanced peak is used for evaluation of lesions, a large part of inflammatory lesions will become false positive. We infer that abundant expansion of capillaries in inflammatory lesions is the reason for the obvious enhancement.
The moderately-highly enhanced ones in this group of lung cancer covered majority, inflammation displayed high enhancement, and tuberculosis, benign tumours were no or low enhanced. CT enhancement degree of lesions was mainly related to the number of vessels necrosis or not, the scope and extent of necrosis and other reasons. In addition, there may be some secondary factors, such as differences of lesion cells, different fibre components in lesions [16]. Littleton et al. studied and found that the enhancement degree of enhancement of lung cancer was squamous cell carcinoma>adenocarcinoma>small cell carcinoma [16]. However, after the comparison, Yamashita et al. believed that adenocarcinoma>large cell carcinoma>squamous cell carcinoma. Thus, we think that the enhancement degree of lung cancer may be related to its tissue types and can reflect the richness degree of vascular in lesions and difference of blood perfusion level in different tissues, which is a main basis of CT enhanced examination judging lung cancer.
The relationship between CT enhancement and immunohistochemistry study showed that the basic conditions for the growth and metastasis of tumour is angiogenesis of tumour, inflammatory lesions, benign tumour, small blood vessels in tuberculosis are inflammatory reflection state of pulmonary vascular with normal structure [17]. They can present a proliferation or increasing change, which is significantly different from angiogenesis of lung cancer in quantity and quality. Yamashita et al. study showed that the relevance of enhancement degree of lung cancer with small vessel (0.02 mm<d<0.1 mm) was larger than that with big vessel (d>0.1 mm) and this small blood vessel was supposed to be an angiogenesis within the tumour rather than rest blood vessels of a host [18]. So CT enhanced form based on angiogenesis provides the pathological information of the lesion appearing with tumour angiogenesis generating [19].
In the numerous factors inducing the formation of blood vessels, VEGF is an important factor secreted by tumour cells, and it has the function of increasing vascular permeability and promotes angiogenesis generating [2]. The high expression of VEGF promotes lung cancer tissue to produce large number of capillaries whose diameter are less than 0.1 mm, and basement membrane is not complete, which makes the contrast agent easy to leak into the interstitial outside the blood vessel, increasing the retention of contrast agent in the interstitial. MVD is a quantification index of tumour angiogenesis, which is the count of capillaries and venules with simple method and unified standard. After proposed by Weidner, it was widely used in the study field of tumour blood vessels. This study shows that the correlation between VEGF and MVD exists.
COX-2 is an important rate limiting enzyme in the process of arachidonic acid four being converted into prostaglandin. Studies have shown that it displays enhanced expression in various tumour tissues, and have believed that it is closely related to the formation of tumour angiogenesis, tumorigenesis, invasion and metastasis [20]. In this study, COX-2 and MVD are correlated. Some scholars have pointed out that the one of the action mechanism of COX-2 is that when it expresses, the product of prostaglandin PGE2 increases, so as to stimulate the production of VEGF and induce tumour angiogenesis. In this study, COX-2 and VEGF are also related [21].
Manley et al. suggested that in the pathological specimens of lung cancer with obvious enhancement, it is seen that the central part of the nodules is multi vascular, and the relationship between the two are closely related [22]. Yamashita et al. have studied and found that MVD in lung cancer tissue was significantly related to the maximum CT enhanced peak value [10]. The above scholar’s conclusions are similar to the results of this study, and we use the linear regression analysis to analyse the relationship of CT enhanced peak value of lung cancer and expression of COX-2, VEGF and MVD. Then we conclude the result that CT enhanced peak and their expression has positive correlation. Because of lymph capillary network, vascular growth can directly affect the metastasis of lymph node in cancer. So the tumour invasion, metastasis and prognosis can be judged and implied according to CT enhanced peak.
CT enhanced peak value in this group of adenocarcinoma was high than those of other pathologic types, which was consistent to the results that VEGF, COX-2 and MVD expression in adenocarcinoma was significantly higher than that of squamous cell carcinoma. Under the observation of microscope, it was observed that tumour micro vessel of adenocarcinomaty was concentrated, the tube wall of which was weak with intravascular material easy to exudation; however, tumour blood vessel size and uneven distribution of squamous cell carcinoma with irregular flakes necrosis region without vascular may be the pathological basis of that enhanced peak of adenocarcinoma was higher than that of squamous cell carcinoma.
The study found that COX-2, VEGF and MVD expression in lung cancer was positively correlated with CT enhanced peak, indicating that CT enhancement can provide a method of preoperative non-invasive diagnosis and evaluation of lung cancer for surgical doctors. However, the patients in this study were followed up for a short period, and had not yet revealed direct correlation between CT enhanced peak value, the expression of three immunological indexes and prognosis of patients, which needs further study.
References
- Folkman J, Beckner K. Angiogenesis imaging. Acad Radiol 2000; 7: 783.
- Hung MS, Chen IC, Lin P Y. Epidermal growth factor receptor mutation enhances expression of vascular endothelial growth factor in lung cancer. Oncol Lett 2016; 12: 4598-4604.
- Wang W, Fan X, Zhang Y. Association between COX-2 polymorphisms and lung cancer risk. Med Sci Monit 2015; 21: 3740-3747.
- Zang J, Hu Y, Xu X. Elevated serum levels of vascular endothelial growth factor predict a poor prognosis of platinum-based chemotherapy in non-small cell lung cancer. Oncotargets Ther 2017; 18: 409-415.
- Maehana S, Nakamura M, Ogawa F. Suppression of lymph angiogenesis by soluble vascular endothelial growth factor receptor-2 in a mouse lung cancer model. Biomed Pharmacother 2016; 84: 660-665.
- Grimminger PP, Stohlmacher J, Vallbohmer Dl. Prognostic significance and clinicopathological associations of COX-2 SNP in patients with non-small. Cell Lung Cancer J Oncol 2009; 139590.
- Lim BJ, Jung SS, Choi SY, Lee CS. Expression of metastasis-associated molecules in non-small cell lung cancer and their prognostic significance. Mol Med Rep 2010; 3: 43-49.
- Lee JM, Mao JT, Krysan K, Dubinett SM. Significance of cyclooxygenase-2 in prognosis, targeted therapy and chemoprevention of NSCLC. Future Oncol 2007; 3: 149-153.
- Li C, Pan T, Li J, Wei X, Chen T, Hu M, Wang Y. Study of COX-2 expression and angiogenesis in non-small cell lung cancer. Chin J Lung Cancer 2004; 7: 501.
- Wang J, Chen J, Guo Y, Wang B, Chu H. Strategies targeting angiogenesis in advanced non-small cell lung cancer. Oncotarget 2017.
- Ma J, Yang YL, Wang Y. Relationship between computed tomography morphology and prognosis of patients with stage I non-small cell lung cancer. Oncotargets Ther 2017; 10: 2249-2256.
- Wang BY, Li YS, Huang GS. The technology of pathology. People's Med Pub House 2000; 365.
- Weidner N. Intratumor micro vessel density as a prognostic factor in cancer. Am J Patho1 1995; 147: 9.
- Zhang M, Kono M. Solitary pulmonary nodules: evaluation of blood flow patterns with dynamic CT. Radiol 1997; 205: 471-478.
- Zeng Q, Xie N, Deng S. Dynamic enhanced CT evaluation of solitary pulmonary nodule. Chinese J Radiol 1997; 31: 164.
- Littleton JT, Durizch ML, Moeller G, Herbert DE. Pulmonary masses: contrast enhancement. Radiol 1990; 177: 861.
- Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T. Correlation between express of vascular endothelial growth factor and tumour vascularity and patient outcome in human gastric carcinoma. J Clin Oncol 1997; 15: 826.
- Yamashita K, Matsunobe S, Takahashi R, Tsuda T, Matsumoto K, Miki H, Oyanagi H, Konishi J. Small peripheral lung carcinoma a evaluated with incremental dynamic CT: radiologic-pathologic correlation. Radiol 1995; 196: 401.
- Miles KA. Functional computed tomography in oncology. Eur J Cancer 2002; 38: 2079.
- Joo YE, Rew JS, Seo YH, Choi YK, Kim YJ, Park CS, Kim SJ. Cyclooxygenase-2 over expression correlates with vascular endothelial growth factor expression and tumour angiogenesis in gastric cancer. J Clin Gastroenterol 2003; 37: 28.
- Davies G, Salter J, Hills M, Martin LA, Sacks N, Dowsett M. Correlation between cyclooxygenase-2 expression and angiogenesis in human breast cancer. Clin Cancer Res 2000; 9: 2651.
- Manley E Jr., Waxman DJ. H460 non-small cell lung cancer stem-like holoclones yield tumours with increased vascularity. Cancer Lett 2014; 346: 63-73.