Research Article - Journal of Clinical Ophthalmology (2021) Volume 5, Issue 3
Study on efficacy of preoperative use of Flurbiprofen and Nepafenac eye drops in maintaining mydriasis during cataract surgery in comparison with duration of surgery in patients with senile cataract: A prospective randomized study
Ambati Divya1, Nareddy Prasanna1*, Kiran Kumar Gudivada2, Sreedhar Wudaru3
1Department of Ophthalmology, Santhiram Medical College and Health Sciences, Nandyala, Andhra Pradesh, India
2Department of Anesthesiology and Critical care, All India Medical sciences, Bibinagar, Hyderabad, Telangana, India
3Department of Critical Care Medicine, Santhiram Medical College and Health Sciences, Nandyala, Andhra Pradesh, India
- Corresponding Author:
- Nareddy Prasanna
Department of Ophthalmology
Santhiram Medical College and Health Sciences
Nandyala
Andhra Pradesh
India
E-mail: prasannan.08@gmail.com
Accepted date: 22 May, 2021
Citation:Divya A, Prasanna N, Gudivada KK, et al. Study on efficacy of preoperative use of Flurbiprofen and Nepafenac eye drops in maintaining mydriasis during cataract surgery in comparison with duration of surgery in patients with senile cataract: A prospective randomized study. J Clin Ophthalmol 2021;5(3):417-421.
Abstract
Objective: Cataract surgery is the predominant procedure in day to day practice for many ophthalmologists. Maintaining appropriate mydriasis during surgery is the key for successful and safe surgery. Cataract surgery initiates the inflammatory cascade, thereby releasing prostaglandins. Prostaglandins lead to constriction of pupil during surgery. Topical NSAIDS are potent inhibitors of cyclooxygenase enzymes thereby inhibiting the biosynthesis of prostaglandins. Aim of the study is to compare the effectiveness of preoperative use of topical Nepafenac 0.1% and Flurbiprofen 0.03% eye drops in sustaining the mydriasis during surgery and comparison of efficacy of both in correlation with the duration of surgery.
Methods: We performed a Prospective, randomized, double blind, comparative study on 100 patients. Patients were randomly allocated into 2 equal groups to receive either Nepafenac or Flurbiprofen. Drug was allocated on the same day and prior to small incision cataract surgery. Pupil diameter was measured at the beginning and at the end of surgery and also duration of surgery was noted. The values of pupil diameter of both groups were compared and also with duration of surgery.
Results: A total of 100 eyes of cataract surgery patients, 46 males and 54 females with mean age group of 62.04 of were included in the study. The mean horizontal (p-value=0.404) and vertical (pvalue= 0.279) diameter of Nepafenac and Flurbiprofen groups were almost similar with no statistically significant difference at the beginning and end of the surgery. There is not a statistically significant difference in variance of mean difference in pupil diameter among different time intervals. But there is a statistically significant difference in variance in mean deviation among patients in Nepafenac group (p-value=0.0089).
Conclusion: Topical Nepafenac and Flurbiprofen are equally effective in maintaining mydriasis, but Nepafenac is more efficacious in maintaining mydriasis as the duration of surgery is increased.
Keywords
Intraoperative, Intraocular lens, Blindness, Surgery.
Introduction
Cataract is the major cause of avoidable blindness worldwide, accountable for 47.8% of blindness, over 17.7 million blind people [1]. In India, cataract is responsible for 50%-80% of bilateral blindness [2]. Annually 3.8 million people become blind because of cataract in India [3]. After all, cataract is the most common cause of blindness, cataract surgery has become the prime concern in the global initiative-VISION 2020 “The Right to Sight” which is dedicated to eliminate avoidable blindness, by increasing the cataract surgery rate and its quality to attain good visual outcomes and enhance the quality of life by year 2020 [3]. Inspite of advances in cataract surgery such as Phacoemulsification has become the preferred technique in developed countries however small incision cataract surgery still remains as a preferred method in developing countries where cataract accounts for majority of the blindness [4].
During cataract surgery, intraocular tissue injury leads to activation of Phospholipase A2 and causes release of inflammatory mediators such as Prostaglandins and Leukotrienes. These endogenous prostaglandins induce miosis during surgery, increased permeability of blood aqueous barrier, conjunctival hyperemia, postoperative inflammation and intraocular pressure changes [5].
The reduction in pupil diameter can lead to more difficult surgery and the risk of surgical trauma, posterior capsule tear and postoperative inflammation is also increased.
A study report showed that mydriasis larger than 6 mm during surgery has lessened the incidence of posterior capsule tear by half. Therefore, to ensure a safe cataract surgery, maintaining an adequate mydriasis is considered important [6]. Topical ophthalmic NSAIDS play a major role in decreasing the release of prostaglandins by inhibiting cyclooxygenase enzyme [7]. Flurbiprofen is the first drug approved by FDA in 1988 used for maintaining intraoperative mydriasis [8]. Earlier studies have mentioned the efficacy of various topical NSAIDS in maintaining mydriasis during cataract surgery.
Newer topical NSAIDS like Nepafenac also showed promising effects. It is a prodrug, which inhibits cyclooxygenase enzyme. It has better corneal penetration and higher ocular bioavailability, resulting in target specific NSAID for prostaglandin inhibition [9]. Not many studies have been conducted in India comparing Flurbiprofen and Nepafenac. This is an efficacy study comparing topical Nepafenac and Flurbiprofen in maintaining mydriasis during cataract surgery in relation to duration of surgery.
Materials and Methods
The study is a randomized, prospective, double blind, single centre, longitudinal and comparative study conducted in patients undergoing small incision cataract surgery at a tertiary care hospital, South India. Approval of the ethics committee of the institution was taken. The study was conducted from August 2020 to December 2020. 100 patients who met the inclusion and exclusion criteria were taken as the sample size.
Inclusion criteria
• Patients age ≥ 50
• Patients diagnosed with senile cataract (irrespective of grade of cataract)
• Patients who underwent small incision cataract surgery with IOL implantation
Exclusion criteria
• Age <50 years
• History of trauma or any previous ocular surgeries in the operating eye
• Glaucoma or active ocular inflammation
• Ocular surface disease and viral keratoconjunctivitis
• History of diabetes mellitus and hypertension
• Use of topical or systemic steroids within 30 days prior to surgery.
• Use of any other topical medications within 30 days prior to surgery (except lubricants)
• Allergy or hypersensitivity to topical NSAIDS and its preservatives
• Intraoperative complications like iridodialysis, PCR, vitreous loss, premature entry or hyphema.
The study details were thoroughly explained to the patients and their relatives and informed consent was taken.
Thorough ophthalmic evaluation was done prior to surgery in all patients and proper history was taken especially medications regarding Benign prostrate hypertrophy as they cause floppy iris. Best corrected visual acuity using Snellen's chart, slit lamp biomicroscopy, IOP by non-contact tonometry, and dilated fundus examination was done. Cataract surgery consent was obtained from all patients. All the patients who met the inclusion criteria were randomly allotted in the two groups A and B with 56 patients in group A and 44 patients in group B.
Group A received 0.1% Nepafenac eye drops and group B received 0.03% Flurbiprofen eye drops. Patients in each group received drops 1 hour prior to surgery with one drop every 15 mins intervals and the last drop being administered 5 mins before peribulbar block. Mydriatic combination of tropicamide 0.8% and phenylephrine 5% was administered 1 hour prior to surgery with 15 mins interval, last drop was installed 10 mins before giving block in all the patients. There was a 5 mins gap during installation of two drops.
All the patients received moxifloxacin 0.5% eye drops 2 days prior to surgery and two times on the day of surgery. All patients underwent small incision cataract surgery with intraocular lens implantation under peribulbar anesthesia using lidocaine 2%, bupivacaine 5%, adrenaline 1 in 10,000 and sodium hyaluronidase. The horizontal and vertical pupil diameter measurements were taken before entry into anterior chamber and at the end of surgery. The measurements of all patients were taken using castroviejo’s callipers placed in front of the cornea, under the same microscope, using the same magnification by different surgeons. The duration of surgery was noted.
The other data collected were age, gender, laterality of eye being operated and the group of drops which they were receiving. The collected data was analyzed using SPSS version 20. Significance is assessed as p<0.05 and a linear regression analysis was done. Student t test has been used to find out the significance of study parameters on a continuous scale between two groups.
Results
Total 100 patients were included in the study. Patients were randomly allocated in each group with 56 in group A and 44 in group B. Table 1 shows the demographic profile of the two groups. There was no statistically significant difference in age, gender and laterality of eye in the two groups.
| Parameters | Nepafenac | Flurbiprofen |
|---|---|---|
| Age | ||
| Mean ± SD | 61 ± 7.41007 | 63.36 ± 7.97989 |
| Gender | ||
| Male | 30 | 15 |
| Female | 26 | 29 |
| Eye | ||
| Right eye | 24 | 19 |
| Left eye | 32 | 25 |
Table 1: Demographic details of the patients.
Table 2 shows pupil diameter. The average horizontal pupil diameter at the beginning of surgery is almost the same in both groups (Nepafenac-7.89 ± 0.94 mm and Flurbiprofen-7.91 ± 0.74 mm). The average vertical diameter at the beginning of surgery in the Nepafenac group was 8.11 ± 1.05 mm and in the Flurbiprofen group was 7.77 ± 0.80 mm. There is no statistically significant difference in horizontal (p-value=0.926) and vertical diameter (p-value=0.085) at the beginning of surgery in both groups. There is no statistically significant difference in horizontal and vertical pupil diameter at the end of surgery in both groups (horizontal p-value=0.946 and vertical p-value=0.912).
| Drug | N | Mean | Std. deviation | p-value | |
|---|---|---|---|---|---|
| Before AC entry H | N | 56 | 7.89 | 0.94 | 0.926 NS |
| U | 44 | 7.91 | 0.74 | ||
| Before AC entry V | N | 56 | 8.11 | 1.05 | 0.085 NS |
| U | 44 | 7.77 | 0.8 | ||
| End of surgery H | N | 56 | 5.25 | 1.99 | 0.946 NS |
| U | 44 | 5.23 | 1.09 | ||
| End of sx V | N | 56 | 5.14 | 2.04 | 0.912 NS |
| U | 44 | 5.18 | 1.28 |
Table 2: Pupil diameter at different stages of surgery (mean, SD) in both groups.
Table 3 shows baseline change in horizontal and vertical pupil diameters from beginning to end of the surgery with percentage loss of mydriasis in both groups. There was no significant difference in baseline change in horizontal diameter (p-value=0.404) and vertical diameter (p-value=0.485) in both groups. At the end of surgery, there was no significant percentage loss of horizontal (p-value=0.485) and vertical (pvalue= 0.311) pupil diameter in both groups.
| Drug | N | Mean | Std. deviation | p-value | |
|---|---|---|---|---|---|
| Base line change horizontal | N | 56 | 2.82 | 1.55 | 0.404 NS |
| U | 44 | 2.59 | 1.08 | ||
| % Loss horizontal | N | 56 | -35 | 19.43 | 0.485 NS |
| U | 44 | -32.59 | 13.41 | ||
| Base line change vertical | N | 56 | 2.86 | 1.54 | 0.279 NS |
| U | 44 | 2.55 | 1.24 | ||
| % Loss vertical | N | 56 | -36.14 | 19.22 | 0.311 NS |
| U | 44 | -32.59 | 14.55 |
Table 3: Baseline change and percentage loss of pupil diameter in both groups.
Table 4 shows the difference of mean horizontal and vertical (H+V) pupil diameters at the beginning and end of surgery in Nepafenac and Flurbiprofen groups in correlation with duration of surgery. Duration of surgery was divided into 3 time intervals, less than 30 mins, 30-60 mins and more than 60 mins. There was no statistically significant difference in the mean value as the duration of surgery is increased in both groups.
| Duration of surgery | Difference of mean of (H+V) before and after surgery in patients receiving nepafenac | Difference of mean of (H+V) before and after surgery in patients receiving | p-value |
|---|---|---|---|
| Flurbiprofen | |||
| Less than 30 mins, (n=72), mean and se | 2.460526 | 2.485294 | 0.9440 (ttest) |
| 0.2891664 | 0.1831047 | 0.9150 (mwu) | |
| 30-60 mins, (n=18) | 3.666667 | 2.583333 | 0.0248(ttest) |
| 0.2161416 | 0.4503085 | 0.0222(mwu) | |
| More than 60 mins, (n=10) | 3.083333 | 3 | 0.8978(ttest) |
| 0.3456074 | 0.5773503 | 0.7333(mwu) |
Table 4: Mean difference of pupil diameter at different time intervals in both groups.
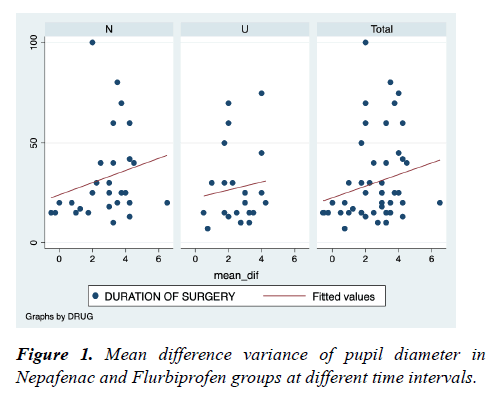
Figure 1 shows mean difference variance of pupil diameter in Nepafenac and Flurbiprofen groups at different time intervals. There is no significant difference in both groups (spearman's rho-Nepafenac group-0.3465 and Flurbiprofen group-0.0749) at different time intervals. But there is a significant difference in variance of mean difference among patients in the Nepafenac group as the duration of surgery is increased (pvalue= 0.0089), but not among patients in the Flurbiprofen group (p-value=0.0749).
Discussion
Mydriasis is the key point for a successful cataract surgery. Pupillary constriction during surgery causes various difficulties like difficulty in nucleus prolapse and makes the eye at risk for further complications due to poor visualization [10]. This risk is increased especially when beginners are performing surgeries during their learning period. Topical NSAIDS are important in combating this situation and also to reduce postoperative pain and inflammation [10-13]. Many comparative studies were done between different NSAIDS and placebo regarding maintaining mydriasis during surgery (Table 5).
| Year | Authors | Drugs compared | Observed effect | Type of surgery | Result |
|---|---|---|---|---|---|
| 2019 | Minj et al. [14] | Nepafenac | Mydriasis | SICS, phacoemulsification | Both are equal |
| Bromfenac | Postoperative inflammation | ||||
| 2018 | Prakash et al. [5] | Nepafenac Flurbiprofen | Mydriasis | SICS | Both are equal |
| 2017 | Chen et al. [13] | Bromfenac | Mydriasis | FLACS | Bromfenac is effective |
| Control group | Postoperative inflammation | ||||
| 2016 | Sharma et al. [10] | Dexamethasone | Mydriasis, | SICS | Ketorolac is more efficacious |
| ketorolac | Postoperative pain and inflammation | ||||
| 2015 | Sarkar et al. [4] | Nepafenac | Mydriasis | SICS | Both are equal |
| Flurbiprofen | |||||
| 2015 | Bansal et al. [11] | Nepafenac | Mydriasis | - | Both are equal |
| Bromfenac | |||||
| 2015 | Jung [15] | Bromfenac | Mydriasis | Phacoemulsification | Both are equal |
| Ketorolac | Postoperative inflammation | ||||
| Control group | |||||
| 2012 | Zanetti et al. [12] | Prednisolone, Ketorolac, Nepafenac and placebo | Mydriasis | Phacoemulsification | Prednisolone, ketorolac and nepafenac are equally efficacious and more than placebo |
| 2011 | Atanis et al. [16] | Ketorolac | Mydriasis | Phacoemulsification | Nepafenac is more efficacious |
| Nepafenac | |||||
| 2011 | Abdel et al. [17] | Flurbiprofen | Mydriasis | - | Both are equal but Flurbiprofen has prolonged action |
| Dexamethasone | |||||
| prolonged action |
Table 5: Comparison of different drugs in various studies.
The results of present study show that nepafenac and flurbiprofen are equally efficacious which corresponds with the other studies in maintaining mydriasis during cataract surgery. The additional information that we obtain from this study is drug efficacy in correlation with duration of surgery which no study has done till now.
Conclusion
In this study, both drugs were equally effective at different time intervals but as the duration of surgery was prolonged, nepafenac had a significant effect in maintaining mydriasis. The prime importance of the study is to observe the effect of the drugs in cataract surgeries with long duration where trainees are the surgeons. As the trainees need sufficient time for the completion of the surgery in their learning phase, the use of NSAIDS preoperatively plays a major role in success of the surgery without any complications. The major limitation of the study is less number of subjects, future research is needed with a large number of study subjects.
References
- Singh S, Pardhan S, Kulothungan V, et al. The prevalence and risk factors for cataract in rural and urban India. Indian J Ophthalmol. 2019;67:477-83.
- Lahane TP. Tackling the cataract backlog – An initiative by the Maharashtra State, India. Indian J Ophthalmol. 2018;66:1391-3.
- Sobti S, Sahni B, Bala K. Surgical coverage of cataract in a rural area of north India: A cross-sectional study. J Family Med Prim Care. 2020;9:4112-7.
- Sarkar S, Mondal KK, Roy SS, et al. Comparison of preoperative nepafenac (0.1%) and flurbiprofen (0.03%) eye drops in maintaining mydriasis during small incision cataract surgery in patients with senile cataract: A randomized, double-blind study. Indian J Pharmacol. 2015;47:491-5.
- Prakash S, Bhandare B, Satyanarayana V, et al. A comparative study on efficacy of nepafenac and flurbiprofen in maintenance of intraoperative mydriasis during cataract surgery: an open label randomized controlled trial. Int J Basic Clin Pharmacol. 2018;7:617-21.
- Guzek JP, Holm M, Cotter JB, et al. Risk factors for intraoperative complications in 1000 extracapsular cataract cases. Ophthalmology. 1987;94:461-6.
- Podos SM. Prostaglandins, nonsteroidal anti-inflammatory agents and eye disease. Trans Am Ophthalmol Soc. 1976;74:637-60.
- Hoffman RS, Braga-Mele R, Donaldson K, et al. Cataract surgery and nonsteroidal antiinflammatory drugs. J Cataract Refract Surg. 2016 Sep;42(9):1368-79.
- Walters T, Raizman M, Ernest P, et al. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J Cataract Refract Surg. 2007;33:1539-45.
- Sharma AK, Sharma HR, Sharma R, et al. A comparison of preoperative topical dexamethasone phosphate versus ketorolac tromethamine in maintaining intraoperative mydriasis during small incision cataract surgery. J Clin Diagn Res. 2016;10:NC09-13.
- Bansal G, Gupta S, Kumar S, et al. Comparison of the effect of topical bromfenac with nepafenac in maintaining mydriasis during cataract surgery. DJO 2015;26:97-100.
- Zanetti FR, Fulco EA, Chaves FR, et al. Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: a randomized trial. Indian J Ophthalmol. 2012;60:277-81.
- Chen H, Lin H, Chen W, et al. Topical 0.1% bromfenac sodium for intraoperative miosis prevention and prostaglandin E2 inhibition in femtosecond laser-assisted cataract surgery. J Ocul Pharmacol Ther. 2017;33:193-201.
- Minj A, Satapathy J, Kumar A. The importance of preoperative topical non-steroidal antiinflammatory agents in cataract surgery-an open label prospective randomised comparative study. Trop J Ophthalmol Otolaryngol. 2019;4:334-40.
- Jung JW, Chung BH, Kim EK, et al. The effects of two non-steroidal anti-inflammatory drugs, bromfenac 0.1% and ketorolac 0.45%, on cataract surgery. Yonsei Med J. 2015;56:1671-7.
- Atanis R, Tuaño PM, Vicencio J, et al. Effect of topical ketorolac tromethamine and topical nepafenac on maintaining pupillary dilation during phacoemulsification. Philipp J Ophthalmol. 2011;36:23–7.
- Abdel M, Mahdy S. Effect of flurbiprofen and dexamethasone acetate in prevention of surgically induced miosis during cataract surgery. J Am Sci. 2011;7:474–8.