Research Article - Biomedical Research (2017) Volume 28, Issue 21
Study on changes of Fas, p-BAD and caspase-8 expression in acute myocardial infarction rats treated by Guanxin decoction
Fawei Yuan and Hao Xia*
Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan 435003, PR China
- *Corresponding Author:
- Hao Xia
Department of Cardiology
Renmin Hospital of Wuhan University, PR China
Accepted on September 14, 2017
Abstract
Objective: To study the expression change of Fas, p-BAD and caspase-8 in rats with Acute Myocardial Infarction (AMI) treated with Guanxin decoction.
Methods: 45 male SD rats were randomly divided into control group, decoction group and shamoperated group. The sham-operated group were treated only with thread without ligation, while rat model of acute myocardial infarction in the other 2 groups were made by ligating the left anterior descending coronary artery, arched ST segment elevation in electrocardiogram suggested that the model was successful. The decoction group was treated with Guanxin decoction 14 g/kg daily, the shamoperated group and the control group were given distilled water 2 ml/d at the same time. The expression change of apoptotic genes Fas, p-BAD and caspase-8 in the sham-operated group (10 rats), the mixture group (13 rats) and the control group (10 rats) were compared.
Results: After the acute myocardial infarction, the positive expression of Fas, p-BAD and caspase-8 decreased in the decoction group, and the difference was statistically significant compared with the model group (t=4.885, P=0.036; t=7.115, P=0.001; t=5.003, P=0.044).
Conclusion: Guanxin decoction can reduce the positive expression of Fas, p-BAD and caspase-8, and improve the cardiac function of myocardial infarction rats.
Keywords
Guanxin decoction, Acute myocardial infarction, Cardiac function, Fas, p-BAD, Caspase-8.
Introduction
Acute Myocardial Infarction (AMI) is a critical medicine emergency, because coronary artery occlusion and blood flow interruption will cause part of myocardium prolonged ischemia and partial necrosis, leading to acute myocardial infarction. The Acute Myocardial Infarction (AMI) is one of coronary heart disease, which is frequently seen clinically, and is mainly manifested as extreme myocardial ischemia, so that acute thrombus may cause obstruction of blood vessels. In recent years, its incidence had been going up predominantly. At present, as the death toll of AMI accounts for a half of cardiovascular diseases all over the world, it has become a top killer of human being. AMI onset is acute with quick changing of illness conditions. Among most aged people, myocardial infarction may easily result in myocardial reconstruction and finally heart failure. Current studies indicate that, during early stage of myocardial infarction, cardiac muscle cell apoptosis plays an important role in myocardial reconstruction and heart failure caused by myocardial infarction and is significant in inhibiting myocardial damage by blocking AMI myocardial infarction. According to WHO report, in recent years, the incidence of the disease is increasing at an average annual rate of 4.32% [1].
Acute myocardial infarction is a common disabling and fatal disease. Although there are many advances in diagnosis and treatment, myocardial cell apoptosis or necrosis, leading to myocardial regional activities of myocardial cells decreased or absent after acute myocardial infarction, is a clinical basis for myocardial infarction clinical manifestations, and also one of the signs of myocardial infarction [2-4]. The study shows that apoptosis is the main reason of myocardial cell loss in early AMI. The apoptotic cardiac myocytes can be detected in the infarct area at 2h after rat coronary artery occlusion, after 5 h the number of apoptotic cells reached to the peak, then gradually reduced [5]. The number of failing myoblasts was significantly less than that of apoptotic cells, which accounted for 86% of all cells lost in the infarct zone. At present, myocardial cells are not reproducible, and the cardiac function is closely related to the number of myocardial cells after myocardial infarction [6-9]. How to protect the myocardium and reduce myocardial infarct size to inhibit ischemia myocardial apoptosis have a very important significance in improving heart function and the prognosis of myocardial infarction. Our previous studies suggested that Guanxin decoction may have a protective effect on ischemic myocardium. The results of this study show that when the anterior descending coronary artery ligation, infarct size in model group was significant greater than that in the control group, while Guanxin decoction group can reduce ischemia induced myocardial infarction in a dose-dependent manner [10]. TUNEL staining is a rapid and convenient method for quantitative detection of apoptosis. It is a sensitive method for detecting tissue apoptosis, used for paraffin embedded tissues with good practicability and high reliability [11-13].
The mitochondrial pathway is one of the main pathways involved in myocardial cell apoptosis, consisting of Bcl-2 family members Bcl-2, Bad and others. These proteins are activated by intracellular signal after death [14]. These Bcl-2 family members containing BH3 domain and other members of the Bcl-2 family such as Bax are mainly loose combined in the outer mitochondrial membrane surface or in cytoplasm, resulting in the oligomerization and inserted into the mitochondrial membrane, causing mitochondrial permeability changes, transmembrane potential loss, cytochrome C and other proteins. The released cytochrome C is binding to Apaf-1 (Apoptosis promoting factor), leading to Apaf-1 oligomers for recruiting and activating caspase family. Caspase-8 is involved in apoptosis execution and induces caspase-3 activation, and the initiation caspase activates the caspase cascade, and then activates the caspase cleavage specific substrate to cause apoptosis [15-18].
Guanxin decoction made of high-dose Astragalus Shixiao powder can improve the myocardial blood supply, effectively relieve chest tightness, chest pain, shortness of breath, palpitations, dizziness and other symptoms, and mainly used for the treatment of myocardial ischemia in coronary heart disease [19,20]. Studies have shown that Guanxin decoction can protect the ischemic myocardium. In this study, the effect of Guanxin decoction on cardiac function in Acute Myocardial Infarction (AMI) was observed by the use of Guanxin decoction in the treatment of acute myocardial infarction in rats, and the changes of Fas, p-BAD and Caspase-8 protein expressions.
Materials and Equipment
Animals
The body mass of male SD rats provided by Zhejiang experimental animal center was between 180~220 g.
Drugs and reagents
Guanxin decoction (composition: 30 g of Huangqi, 20 g of Puhuang, 20 g of Wulingzhi) was condensed into 2 g/ml crude drug decoction, provided by the drug manufacturing room of Wuxi Hospital of traditional Chinese medicine.
Primary antibody diluent and HRP labeled secondary antibody were from Shanghai Mingrui biotech Co. Ltd.; Fas, p-BAD and caspase-8 mouse monoclonal antibody, FasL polyclonal antibody were purchased from Bangfei Biotech Co. Ltd.; Protein Quantification Kit was provided by Beijing pulilai Gene Technology Co Ltd; TUNEL Kit was from Shanghai Bogu biological company.
AMI group
White rate are abdominally injected with 10% chloral hydrate for chloralization, hairs on neck and chest of white rate should be cleaned and povidone is used to sterilize neck skin to make a 1 cm incision so as to expose trachea for connecting a respirator. The parameters of the respirator are adjusted to: respiratory frequency: 120 times/min, tidal volume: 7-8 ml and respiratory time ratio: 1:2. After chest skin has been sterilized with povidone, a surgery incision is made at the 4th left rib on the chest and the skin is dissected layer by layer to fully expose the heart; the left anterior descending branch of coronary artery should be found out between left auricle and pulmonary infumdibulum and then ligated. When it is found that ST section rises up on the Electro-Cardiograph, the rhythm of the heart is relatively slow and the left ventricle turns white, it means that AMI has been made successfully. At last, 36 white rates, of which AMI is successful, are classified as the AMI group at random.
Model preparation
80 SD rats were randomly divided into 5 groups: Guanxin decoction small dose group and model group with 16 rats in each group of Guanxin decoction group at 7 d before the operation began intragastric dose were 14 g/kg daily at the same time, the control group given distilled water 2 ml/d by intragastric administration, rats were anesthetized with experiment animal ventilator for positive pressure breathing, 6-0 non-invasive with needle suture through the left anterior descending coronary artery puncture depth is about 1~2 mm, ligation of the left anterior descending coronary artery caused by acute myocardial infarction model, ECG ST segment elevation was arched suggesting that the model was successful. The coronary artery was not ligated in the control group.
TdT-mediated dUTP nick end labeling (TUNEL)
In 4.5 h after the TdT-mediated dUTP nick end labeling (TUNEL) is adopted to determine apoptosis, the heart is taken out, myocardial tissue below the ligature location is fixed with 4% paraformaldehyde, embedded with common paraffin and then sliced. All operations should be done according to the insert of TUNEL test kit of ROCHE Company. Each paraffin block should be sliced and 6 viewpoints are taken to shoot each slice to count the number of apoptosis cells and total number of the cells. The images are analysed with Image Pro Plus 6.0 software. The fixed unit of enumeration for apoptosis cells is expressed in apoptosis index in the following equation: apoptosis index=number of apoptosis cell/total numbers of observed cells × 100 %.
Index detection
The rat left ventricular myocardium in infarcted zone was taken for nitrogen trituration, then adding lysate. After homogenate centrifugation, the protein concentration of supernatant was determined by BCA assay, then degenerated and kept at -80°C. 50 μg from each sample was taken for 12% SDS-PAGE and then transferred to PVDF membrane, 5% skim milk was used to close 1.5 h at room temperature in a shaker. Fas, FasL diluted with primary body diluent were added for incubation overnight. HRP was added for labelling (1:1000 dilution), ECL was used for color, the film was scanned firstly, then Image J was employed for analysis, β-actin diluted in 1:1000 as a control, the ratio means the relative content of protein expressed.
Statistical analysis
The experimental data were analysed with SPSS 19.0. The measurement data were present as mean ± standard deviation (͞x ± s). LSD method was used for pairwise comparison. In the case of the normal distribution and homogeneity, the single factor analysis of variance was applied, P<0.05 means the difference was statistically significant.
Results
General situation
The activity, reaction sensitivity fur luster and diet of the rats in Guanxin decoction group were improved at different extend than those in the model group; the activity of rats in the control group is good, their fur was neat and shiny, and have sensitive reaction and normal diet, while the rats in the model group had poor activity, slow response, uneven fur, dull skin and less diet.
Effect of Guanxin decoction on apoptosis in rats with myocardial infarction
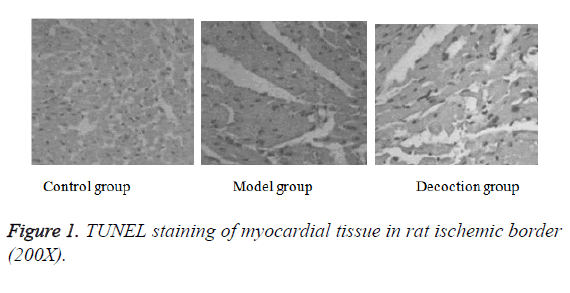
After in situ end labeling, negative nuclear staining was blue in slices of control group, while positive nuclear staining was brown in the slices of model group. The size of the nucleus is reduced or shrunk obviously, and the shape is round. A large amount of positive nuclear were observed in myocardial tissue slice of model group, with the increased apoptosis index, up to (57.6 ± 11.2%), which had a significant difference compared with the control group (P<0.05). Positive nuclear in Guanxin decoction group were rare. The apoptosis index of Guanxin decoction group was (23.1 ± 3.8%), which was significantly lower than that of the model group, with statistically significant difference (P<0.05); the apoptosis index was decreased most obviously (Figure 1).
Effect of Guanxin decoction on the expression of Fas and protein in myocardial infarction rats
Compared with the control group, the Fas expression in the model group increased significantly, with statistical difference (P<0.05); Fas protein expression in decoction group was most significantly decreased (t=4.885, P=0.036) while compared with the model group (Table 1).
| Group | Fas |
|---|---|
| control group | 0.18 ± 0.05* |
| model group | 0.48 ± 0.13# |
| decoction group | 0.19 ± 0.08*# |
Note: compared with model group, *P<0.05; compared with control group, #P<0.05.
Table 1. Effects on the Fas expression in myocardial infarction rats of each group.
Effect of Guanxin decoction on the p-BAD expression in rats with myocardial infarction
Compared with the myocardial infarction model group, the expression of myocardial p-BAD in decoction group was significantly decreased with statistically significant difference (t=7.115, P=0.001); compared with model group, the expression of p-BAD protein in myocardial tissue in myocardial infarction model group was significantly increased, the difference was statistically significant (P<0.05) (Table 2).
| Group | p-BAD |
|---|---|
| Control group | 0.23 ± 0.07* |
| Model group | 0.69 ± 0.15# |
| Decoction group | 0.37 ± 0.12*# |
Note: compared with model group, *P<0.05; compared with control group, #P<0.05.
Table 2. Effects on the p-BAD expression in myocardial infarction rats of each group.
Effect of Guanxin decoction on the caspase-8 expression in rats with myocardial infarction
Compared with the control group, the caspase-8 expression in the myocardial tissue of the myocardial infarction model group increased significantly, the difference was statistically significant (P<0.05). Compared with the myocardial infarction model group, the expression of caspase-8 in the myocardial tissue of the decoction group was significantly decreased, with statistical significance (t=5.003, P=0.044), as shown in Table 3.
| Group | Caspase-8 |
|---|---|
| control group | 0.25 ± 0.05* |
| model group | 0.53 ± 0.12# |
| decoction group | 0.34 ± 0.08*# |
Note: compared with model group, *P<0.05; compared with control group, #P<0.05.
Table 3. Effects on the caspase-8 expression in myocardial infarction rats of each group.
Discussion
After acute myocardial infarction, myocardial apoptosis or necrosis will lead to reduction or deletion of some active myocardial in myocardium, which is the clinical basis of various clinical manifestations of myocardial infarction. Heart has weak regeneration and repair ability, which is more sensitive to many stimuli, especially ischemic [21]. At present, myocardial cells are non-renewable, and the cardiac function is closely related to the number of myocardial cells after myocardial infarction. How to protect the myocardium and reduce the apoptosis of myocardial cells is of great significance for improving the cardiac function and the prognosis of myocardial infarction. Some studies suggest that apoptosis is a major cause of myocardial cell loss in early acute myocardial infarction. The apoptotic cardiac myocytes can be detected in the infarct area at 2 h after rat coronary artery occlusion, after 5 h the number of apoptotic cells reached to the peak, then gradually reduced. Myocardial cells of 2 h can be detected in the infarct area of apoptosis of rat coronary artery occlusion, 5 h after the number of apoptotic cells reached the peak, then decreased gradually. The number of necrotic cardiomyocytes was significantly less than that of apoptotic cells, which accounted for 86% of all cells lost in the infarct zone. TUNEL staining is a rapid and convenient method for quantitative detection of apoptosis. It is a sensitive method for detecting tissue apoptosis, used for paraffin embedded tissues with good practicability and high reliability [22-24].
The pig left anterior descending coronary artery model by incomplete ligation demonstrated that a large number of myocardial cells appeared apoptosis in the myocardial blood flow block area, and increased gradually as the degree of ischemia increased. As the study on cell apoptosis developed, many studies proved that myocardial apoptosis is also involved in the formation of complications of acute myocardial infarction [25]. The experiments suggested that myocardial cells existed apoptosis, after acute myocardial infarction, infarction and infarct zone firstly appeared myocardial cell apoptosis, while myocardial necrosis appeared after myocardial cell apoptosis. The apoptosis of myocardial cells have dynamic changes with the time, the expression was different in different parts of myocardial infarction, affected by myocardial necrosis. So it is believed that the myocardial cell apoptosis in acute myocardial infarction is the main way leading to cell morphology impairment and reduced myocardial parenchymal cells, and the decreased number of parenchymal cells caused by cell apoptosis was more severe than that caused by myocardial necrosis [26-29].
After myocardial infarction, due to decreased number of cardiomyocytes, cardiac fibroblasts proliferation, ventricular wall hypertrophy, increased wall strength, increased mechanical heart load, decreased cardiac contractility, increased expression of oxidation endogenous products and pro apoptotic gene Fas, increased endocrine activity of sympathetic adrenal system, renin-angiotensin-solid acid ketone endocrine system, a high power state compensated heart function, chronic and persistent oxidative metabolism increases to further induce increased oxidative stress, and activate apoptosis and signal transduction of apoptosis gene, finally leading to myocardial cell apoptosis [30]. Rats with acute myocardial infarction and myocardial ischemia model of rats were confirmed: positive apoptotic protein and mRNA expression of pro apoptotic gene Fas increased significantly compared with normal rats. Apoptotic bodies appeared, accompanied with protein hydrolysis and DNA degradation. Fas molecule is one typical type I membrane protein, because they have special structural regions that promote cell death, also known as the death domain. FasL interacts with Fas to initiate apoptotic signaling. The activation step is as follows: firstly, ligand receptor induced trimerization, then apoptosis complex was formed in the cell membrane, this complex contained endogenous death domain associated Fas protein FADD. The death domain activated acidic nerve sheath flow (ASM), and release ceramide, followed with the activation of threonine or serine protein kinase to produce a variety of biological chemical mediators, which activated aspartic acid specific cysteine protease related proteases, such as caspase-3 to initiate apoptosis. With target cell activity of Fas interacted with joint protein of FADD, the death domain of the comlex received apoptotic signals, and passed to the apoptosis protease caspase-8, activate caspase-8 molecules; at the same time cut Bd-2 family protein, induce the release of cytochrome C, so as to activate zymogen caspase-9. At last, cascade reaction activates caspase-3 and cells undergo apoptosis [31,32].
Apoptosis is a programmed death mode controlled by gene and is mainly guided by endogenous and exogenous means, of which the exogenous mean is guided by the death acceptor Fas on cell surfaces. Guanxin decoction can improve cardiac function indices of white rate after myocardial infarction, inhibit apoptosis of myocardial cells in marginal zones of infarction and protect the role of myocardium, but there is less studies on the relation with the expression level of Fas albumen. Whether the mechanism of Guanxin decoction inhibiting apoptosis is related to other signal transduction pathways is still in needs for further study.
Research results indicate that myocardial cell apoptosis is quite little in normal conditions, but increases obviously after myocardial infarction. In various dose groups of Guanxin decoction, myocardial cell apoptosis decreases to different extents, of which the group with great dose of Guanxin decoction has the lowest apoptosis indices, and Guanxin decoction has outstanding protection role for myocardial cell apoptosis, which means that myocardial cell apoptosis possibly has ischemia myocardial preservation. After acute myocardial infarction of white rate, the decoction largely reduces positive expressions of Fas, p-BAD and caspase-8 albumens.
References
- Donoiu I, Istratoaie O. Varicella-zoster myocarditis mimicking acute myocardial infarction. Curr Health Sci J 2014; 40: 78-80.
- Aundhakar SC, Mahajan SK, Mane MB. Reactive thrombocytosis leading to acute myocardial infarction. J Assoc Phys India 2013; 61: 745-747.
- Ritschel VN, Seljeflot I, Arnesen H, Halvorsen S, Weiss T. IL-6 signalling in patients with acute ST-elevation myocardial infarction. Results Immunol 2013; 4: 8-13.
- Chen TL, Zhu GL, He XL. Effects of coronary mixture on hemodynamics, infarction area and apoptosis of acute myocardial infarction rats. Chin J Trad China Med 2013; 12: 2711-2713.
- Jain J, Narang UR, Jain VV. A comparative study of the C-reactive protein and the ST-score (ECG) as prognostic indicators in acute myocardial infarction in a rural resource-constrained hospital setting in central India: a cross-sectional study. Heart View Off J Gulf Heart Assoc 2013; 14: 171-178.
- Zhu GL, Chen QL, Chen TL. Effect of coronary mixture on the expression of myocardial bFGF and angiogenesis in rats with acute myocardial infarction. Zhejiang Zhongxi Med Assoc Magaz 2011; 21: 758-761.
- Zhao QF. Bolted size mixture effect on cardiac function after acute myocardial infarction patients and experimental study of intervention ventricular remodeling. Nanjing Univ Trad Chinese Med 2008.
- Bhuyan SS, Wang Y, Opoku S. Rural-urban differences in acute myocardial infarction mortality: Evidence from Nebraska. J Cardiovasc Dis Res 2013; 4: 209-213.
- Wang B, Han Y L, Li Y. Coronary collateral circulation: Effects on outcomes of acute anterior myocardial infarction after primary percutaneous coronary intervention. J Geriatr Cardiol 2011; 8: 93-98.
- Shyu KG, Wang BW, Cheng WP. MicroRNA-208a increases myocardial endoglin expression and myocardial fibrosis in acute myocardial infarction. Canadian J Cardiol 2015; 31: 679-690.
- Zhu GL, Qian BQ, Zhou F. Effect of coronary mixture on VEGF expression in rats after myocardial infarction. China J Trad China Med 2011; 2: 247-249.
- Cui M, Gong QY, Yao MH. The protective effect of musk mixture on rats and dogs in acute myocardial infarction and its mechanism. China Pharm 2007; 24: 211-214.
- Olson RE, Vojvodic RW, Bettencourt J. Recruiting for acute myocardial infarction cell therapy trials: challenges and best practices for the CCTRN. Clin Res 2014; 28: 71-77.
- Liang HW, Huang YP, Pan SL. Parkinson disease and risk of acute myocardial infarction: a population-based, propensity score-matched, longitudinal follow-up study. Am Heart J 2015; 169: 508-514.
- Rallidis LS, Sakadakis EA, Tympas K. The impact of smoking on long-term outcome of patients with premature (≤ 35 years) ST-segment elevation acute myocardial infarction. Am Heart J 2015; 169: 356-362.
- Begot I, Peixoto TC, Gonzaga LR. A home-based walking program improves erectile dysfunction in men with an acute myocardial infarction. Am J Cardiol 2015; 115: 571-575.
- Reddy VS, Bui QT, Jacobs JR. Relationship between serum low-density lipoprotein cholesterol and in-hospital mortality following acute myocardial infarction (the lipid paradox). Am J Cardiol 2015; 115: 557-562.
- Javadi HR, Allami A, Mohammadi N. Opium dependency and in-hospital outcome of acute myocardial infarction. Med J Islam Repub Iran 2014; 28: 122.
- Mir JU, Raheel Jahangir J, Asfandyar Q. Left ventricular thrombus in patients with acute anterior wall myocardial infarction. J Ayub Med Coll Abbottabad JAMC 2014; 26: 491-495.
- Verma GC, Jain G, Wahid A. Acute ischaemic stroke and acute myocardial infarction occurring together in domestic low-voltage (220-240 V) electrical injury: a rare complication. J Assoc Phys India 2014; 62: 620-603.
- Zuhaid M, Kazmi S, Farooq U. Knowledge of modifiable risk factors of cardiovascular diseases among patients with acute myocardial infarction. J Ayub Med Coll Abbottabad JAMC 2014; 26: 364-367.
- Karbalaie S, Hosseini K, Bozorgi A. The relation of ST segment deviations in 12-lead conventional Electrocardiogram, right and posterior leads with the site of occlusion in acute inferior myocardial infarction. Med J Islam Repub Iran 2014; 28: 103.
- Wei LP, Chen TL, He XL. The effect of astragalus on the cardiac function of acute myocardial infarction and the expression of caspase-8. Chin Med Emerg 2013; 22: 539-540.
- Zhao L, Wei ZH. The effect and significance of atorvastatin on the apoptosis of myocardial cells and caspase-8 activation in rats with coronary arterial microembolization. Chin J Lab Diagn 2011; 6: 1003-1005.
- Studer M, Zuber M, Jamshidi P. Thromboembolic acute myocardial infarction in a congenital double chambered left ventricle. Ind Heart J 2011; 63: 289-290.
- Mohan JC, Shekhar C, Mohan V. Intramyocardial hematoma following primary percutaneous intervention in acute myocardial infarction: realtime 3D echocardiographic imaging. Ind Heart J 2011; 63: 277-278.
- Nagayama M, Itoh H, Maeda T. Cardiac rehabilitation for patients with acute myocardial infarction. Nihon Rinsho Jap J Clin Med 2011; 69: 203-209.
- Nagai T, Yoshikawa T. Diagnostic procedure for acute ST-elevation myocardial infarction. Nihon Rinsho Jap J Clin Med 2011; 69: 126-132.
- Nakatani D, Sakata Y, Sato H. Factors to predict cardiovascular risk in patients with acute myocardial infarction. Nihon Rinsho Jap J Clin Med 2011; 69: 105-110.
- Nik Azlan NM, Mohamad Shazwan A, Nurul Amirah M. Asscociation of risk factors and its bleeding complication for tenecteplase administered in acute myocardial infarction (AMI). Med J Malay 2013; 68: 381-383.
- Shah MJ, Bhatt NR, Dabhi A. A study of 100 cases of arrhythmias in first week of acute myocardial infarction (AMI) in Gujarat: a high risk and previously undocumented population. J Clin Diagn Res 2014; 8: 58-61.
- Olson RE, Vojvodic RW, Bettencourt J. Recruiting for acute myocardial infarction cell therapy trials: challenges and best practices for the CCTRN. Clin Res 2014; 28: 71-77.