Research Article - Current Pediatric Research (2019) Volume 23, Issue 2
Study of balance between T-helper 17 /T-regulatory cells in systemic lupus erythematosus and its relation to disease activity
Sheren Eesam Maher1*, Hanan Aly Taha2, Walaa Hozayn3, Ahmed Mohamed Okasha4, Amer Ahmed3, Manar Ali Shata3 and Emad Abdel-Naem5
1Department of Paediatrics, Minia University, Egypt
2Department of Internal Medicine, Beni-Suef University, Egypt
3Department of Biotechnology and Life Sciences, Beni-Suef University, Egypt
4Department of Biochemistry, Minia University, Egypt
5Department of Clinical pathology, Minia University, Egypt
- *Corresponding Author:
- Sheren Eesam Maher
Assistant Professor of Pediatrics
Minia University, Egypt
Tel: 00201001097818
E-mail: sherenesammaher@yahoo.com
Accepted on March 25th, 2019
Abstract
Background and objectives: Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that causes damage to many organs of the body. The balance between pro-inflammatory T-helper 17(Th17) and regulatory-T (Treg) cells play an important role in the pathogenesis of SLE. The aim of the study is to assess Th17/Treg ratio and their relation to disease activity in both adult and juvenile SLE patients on T -lymphocytes using flow cytometry.
Patients and methods: This study was carried out on 80 patients; 40 patients with adult SLE and 40 patients with juvenile SLE. Forty subjects were taken as control groups. All patients and control were subjected to history taking and measuring of Complete blood count (CBC), C3, C4, Antinuclear antibody (ANA), Anti ds-DNA and protein /creatinine ratio in urine and measurement of Th17 and Treg by flow cytometry.
Results: There was statistically significant increase in Th17/Treg ratio in both active adult and juvenile SLE when compared with inactive adult and juvenile SLE and control groups. Also there was statistically significant increase in Th17/Treg ratio in inactive juvenile SLE when compare with control, however, there was no significant difference between inactive adult SLE when compared with control groups. There was a significant positive correlation between Th17cells and SLEDAI, ANA, serum creatinine in both adult and juvenile SLE, Also,a significant positive correlation between Treg cells and absolute neutrophil count, platelets , C3 and C4 in both adult and juvenile SLE.
Conclusion: Th17/Treg ratio is increased in active adult and juvenile SLE when compared with inactive SLE and control and thus this ratio may be used as a marker of disease activity.
Keywords
Systemic lupus erythematosus (SLE), T-helper 17 cells (Th17 cells), Regulatory-T cells (Treg cells).
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that affects all organs of the human body. The exact cause of SLE is still unknown, but several factors have been associated with disease (e.g. genetics, environment, and sex hormone factors) [1,2].
Th17 cells differentiate from naive CD4+ T cells by stimulation with antigen in the presence of TGF-Beta and IL-6. Th17 cells secret IL-17 and IL-22 [3]. The principal role of Th17 cells appears to be protection against extracellular bacterial and fungal infections through recruitment of neutrophils and other leucocytes. Studies in mice and humans suggested that Th17 cells may be important mediators of tissue damage in immune -mediated inflammatory diseases such as SLE [4,5].
Treg cells represent the other face of the coin in SLE pathogenesis [6]. Treg cells are a subset of CD4+ cells whose function is to suppress the immune response and maintain self-tolerance. The majority of Treg express high levels of CD25 but no other markers of T-cell activation. Treg cells are generated mainly by self-antigen recognition in the thymus but they also develop in the peripheral lymphoid organs. The generation and survival of Treg cells are dependent on TGFB-eta and IL-2 and stimulation by the B7 and CD28 pathway. [7].
Treg cells inhibit activation and effector function of auto reactive T-lymphocytes thus preventing the onset of aberrant self-immune response [8]. The balance between pro-inflammatory Th17 and Treg cells play an important role in the pathogenesis of SLE. The aim of this study to assess the balance between Th17 and Treg cells in SLE and its relation to disease activity in both adult and juvenile SLE patients.
Patients and Methods
This study was carried out at clinical immunology unit, faculty of medicine, Bane Suef University hospital during the period from March 2016 to February 2018. The study included 80 SLE patients who divided into: 40 adult SLE patients classified by using SLICC criteria [9], (18 had active SLE and 22 inactive SLE patients) and 40 juvenile SLE patients classified by using SLICC criteria [10], (15 had active SLE and 25 had inactive SLE patients). The juvenile SLE patients were selected from the pediatric Department, Faculty of Medicine, El-Minia University. The activity was estimated according to systemic lupus erythematous disease activity index (SLEDAI) which consider active >5 [11]. The activity of lupus nephritis was assessed according to renal SLEDAI
Eighty healthy normal 40 adults and 40 children matched in age, gender, and geographical location, were used as the control.
All patients were subjected to history taking, clinical examination and laboratory investigations including CBC, serum creatinene, C3, C4, ANA, Anti ds-DNA and protein / creatinine ratio in urine and measurement of Th17 cells and Treg cells by flow cytometry.
The study was approved by the ethics of the faculty of medicine of Bani Suef University. The written informed consents were obtained from all patients (SLE patients and control). In case of juvenile patients the informed consents were obtained from the parents.
Sampling protocol
Five ml of venous blood was collected by venipuncture and was divided into, 2 ml on EDTA tube for CBC and flow cytometric measurement of Th17 cells and Treg cells, 3 ml of blood on plain tube, allowed to be clotted and the serum was separated by centrifugation at 3000 rpm for assessment of serum creatinine, C3, C4, ANA, and Anti ds-DNA. A random urine sample was collected for assessment of protein/creatinine ratio.
Assay protocol
CBC was by using automated cell count, Sysmex KX-21- N(TAO medical incorporation, Japan) serum creatinine was assessed by chemical auto-analyzer ( SLECTRA PROXL ) ANA and Anti-ds-DNA by using fully automated analytical instrument Alegria, Germany, the kits were supplied by Orgenetic diagnostic GMBH [12,13]. C3 and C4 by using Cobas AS e 411 (ROCHE HITCHI), the kits were supplied by Roche Diagnostics [14]. Albumin in urine was done by turbid metric method and creatinine in urine was done by using chemical auto-analyzer after diluting the urine samples 50 times.
Immunofluorescence staining of Th17 cells
Staining of Th17 was done by using bioligand Human Th17 Flowä Kit (anti-CD3 FITC/anti-CD4 PE/anti-IL-17 Per CP) according to the following protocol : 100 μl of blood was added into two tubes (test and control) and 0.5 ml of fixation reagent was added and incubation at room temperature for 20 minutes then centrifugation. The supernatant was removed. 2 ml of Intracellular Staining permeabilization reagent was added to each tube and incubated at room temperature for 20 minutes, then centrifugation and the supernatant was discarded. 20 μl of IL-17 per CP /CD3 FITC/CD4 PE was added into the test tube only and Isotype control antibody was added in the negative control tube and incubated at room temperature in the dark for 30 minutes. Washing twice with 2 ml Intracellular Staining Perm Wash Buffer, and re-suspend in 0.5 ml PBS and analyzed by flow cytometry [15].
Immunofluorescence staining of Treg cells
Detection of Treg cells was done by using bioligand One Step Staining Human Treg Flowä (FOXP3 FITC/CD25 PE/CD4 PerCP) according to following protocol: 100 μl of blood was added into two tubes (test and control) and 0.5 ml of fixation reagent was added and incubation at room temperature for 20 minutes and centrifugation. The supernatant was removed and 2 ml of Intracellular Staining permeabilization reagent was added to each tube and incubated at room temperature for 20 minutes, then centrifugation and the supernatant was removed. 20 μl of FITC anti-human FOXP3/ CD25 PE/CD4 PERCP was added into the test tube only and Isotype control antibody was added in the negative control tube and incubated at room temperature in the dark for 30 minutes. Washing twice with 2 ml Intracellular Staining Perm Wash Buffer, and re-suspend in 0.5 ml PBS then analyzed by flow cytometry [16].
Statistical Analysis
Statistical analyses were performed using IBM SPSS version 21.0 software. A paired t-test was used to calculate the difference of two parameters in groups. Categorical data were evalua were analyzed using: ted using Chi-square test. P<0.05 was accepted as statistically significant. Two study groups ANOVA test for comparison of quantitative data.
Bivariate correlation analysis for association analysis. Note the ratio Th17/Treg calculated case by case (for each case individually), then the mean was calculated for the calculated ratios.
Results
Table 1 serum creatinine, ANA, Anti ds-DNA, and protein/ creatinine ration were significantly higher in adult and juvenile SLE than controls while C3, C4, absolute neutrophils count and platelet count were significantly lower in adult and juvenile SLE than controls. Table 2 absolute count of lymphocytes, CD4 T-lymphocytes, percentage and absolute count of Treg cells were significantly lower in adult and juvenile SLE when compared with controls while percentage and absolute count of Th17 cells and Th17/Treg ration were significantly higher in adult and juvenile SLE than control.
| Variables | Adult SLE N=40 | Adult Control N=40 | P value | Juvenile SLE N=40 |
Juvenile control N=40 |
P- value |
|---|---|---|---|---|---|---|
| Age (year) | 31.7 ± 8.0 | 36.6 ± 8.7 | 0.69 | 12.5 ± 1.7 | 12.0 ± 1.9 | 0.932 |
| Sex: Female/Male | 30/10 (75 /25%) | 30/10 (75/25%) | 1 | 32/8 (80/20%) | 32/8 (80/20%) | 1 |
| ANA( Index ) | 4.89 ± 2.2 | 0.26 ± 0.12 | <0.001 | 3.94 ± 1.9 | 0.16 ± 0.01 | <0.001 |
| Anti ds DNA (IU/ml ) | 219.5 ± 21.2 | 3.70 ± 0.20 | 0.001 | 185.06 ± 18.00 | 2.5 ± 0.20 | <0.001 |
| C3 (mg/dl ) | 60.0 ± 18.5 | 125.7 ± 12.0 | 0.003 | 72.5 ± 9.5 | 110.0 ± 14.5 | 0.004 |
| C4 ( mg/dl ) | 8.07 ± 5.6 | 29.8 ± 4.4 | 0.004 | 9.1 ± 5.2 | 34.4 ± 4.15 | 0.091 |
| Protein/creatinine ratio(mg/gm creatinine) | 257.4 ± 118.8 | < 30.0 | <0.001 | 190.0 ± 128.0 | <30.0 | <0.001 |
| Neutrophil count(cell/ul) | 2115.0 ± 740.0 | 5020 ± 1100.0 | 0.001 | 1985.0 ± 280.0 | 2990.0 ± 340.0 | <0.001 |
| Platelet count (cell/ul ) | 119.5 ± 41.5 | 293.0 ± 78.0 | 0.001 | 105.5 ± 50.0 | 316.0 ± 94.0 | <0.001 |
| Serum creatinine (mg/dl) | 1.9 ± 0.4 | 1.1 ± 0.2 | 0.001 | 1.4 ± 0.3 | 0.7 ± 0.3 | <0.001 |
Table 1. Demographic and laboratory data of the studied groups.
| Variables | Adult SLE N=40 | Adult Control N=40 | P–value | Juvenile SLE N=40 | Juvenile Control N=40 | P-value |
|---|---|---|---|---|---|---|
| Lymphocytes (cells/uL) | 1434.4 ± 356.03 | 1801.5 ± 115.8 | <0.001 | 2429.7 ± 347.1 | 3650.5 ± 115.8 | <0.001 |
| CD4+ (cells/uL) | 461.3 ± 176.1 | 777.1 ± 81.8 | <0.001 | 831.3 ± 180.2 | 1057.1 ± 81.8 | <0.001 |
| Sub-populations of CD4+ lymphocytes (absolute counts) | ||||||
| Th17 absolute count (cells/uL) | 7.03 ± 0.74 | 5.69 ± 2.1 | 0.011 | 27.03 ± 0.74 | 6.60 ± 2.1 | <0.001 |
| Treg (cells/uL) | 9.53 ± 4.0 | 15.4 ± 2.06 | <0.001 | 8.47 ± 4.3 | 34.4 ± 2.06 | <0.001 |
| Sub-populations of CD4+ lymphocytes (% of CD4+) | ||||||
| Th17 (% of CD4+) | 1.07 ± 0.58 | 0.37 ± 0.13 | <0.001 | 2.9 ± 0.62 | 0.57 ± 0.13 | <0.001 |
| Treg (% of CD4+) | 1.1 ± 0.51 | 1.4 ± 0.18 | 0.019 | 1.0 ± 0.52 | 3.02 ± 0.17 | <0.001 |
| T h17/Treg ratio | 1.64 ± 1.6 | 0.23 ± 0.11 | <0.001 | 1.61 ± 1.57 | 0.24 ± 0.08 | <0.001 |
Table 2. Lymphocyte Subpopulations of the studied groups, Note : the ratio Th17/Treg in the above table was calculated case by case (for each case individually),then the mean was calculated for the calculated ratios.
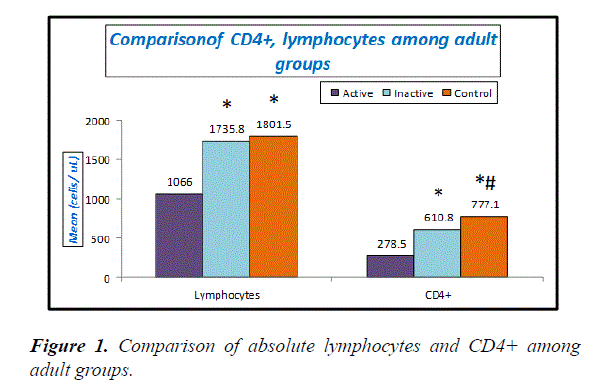
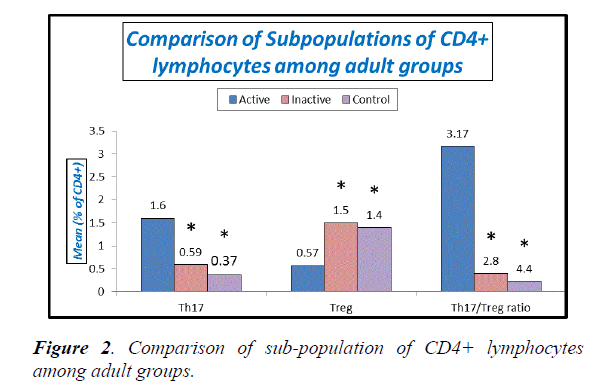
Table 3, Figures 1 and 2 explain absolute count of lymphocytes, absolute count of CD4 cells and Treg cells were significantly lower in active adult SLE when compared with inactive adult SLE and control while Th17 cells and Th17/Treg ratio were significantly higher in active adult SLE than inactive adult SLE and control. No significant difference between inactive adult SLE and control as regard these parameters.
| Variables | Active N=18 | Inactive N=22 | Adult Control N=40 | P-value |
|---|---|---|---|---|
| Lymphocytes (cells/ uL) | 1066 ± 40.8 | 1735.8 ± 36.1 | 1801.5 ± 115.8 | P1: <0.001 |
| P2: <0.001 | ||||
| P3: 0.078 | ||||
| CD4+ (cells/ uL) | 278.5 ± 36.1 | 610.8 ± 57.6 | 777.1 ± 81.8 | P1: <0.001 |
| P2: <0.001 | ||||
| P3: <0.001 | ||||
| Th17 (% of CD4+) | 1.6 ± 0.25 | 0.59 ± 0.18 | 0.37 ± 0.13 | P1: <0.001 |
| P2: <0.001 | ||||
| P3: 0.060 | ||||
| Treg (% of CD4+) | 0.57 ± 0.16 | 1.5 ± 0.18 | 1.4 ± 0.18 | P1: <0.001 |
| P2: <0.001 | ||||
| P3:0.149 | ||||
| T h17/Treg ratio | 3.17 ± 1.43 | 0.39 ± 0.12 | 0.23 ± 0.11 | P1: <0.001 |
| P2: <0.001 | ||||
| P3:0.452 |
Table 3. Comparison between lymphocytes, CD4+, Th17, Treg and Th17/Treg ratio among active and inactive adult SLE patients and control, Note the ratio Th17/Treg in the above table was calculated case by case (for each case individually), then the mean was calculated for the calculated ratios. P1: p-value between active SLE patients and control. P2: p-value between active and inactive SLE patients. P3: p-value between inactive SLE patients and control.
Table 4 explain absolute count of lymphocytes, absolute count of CD4 cells and Treg cells were significantly lower in active and inactive juvenile SLE when compared with control while Th17 cells and Th17/Treg ratio were significantly higher in active and inactive juvenile SLE than control.
| Variables | Active N0=18 | Inactive NO=22 | Control N0=40 | P-value |
|---|---|---|---|---|
| Lymphocytes | 1167.3 ± 39.6 | 2726.2 ± 113.1 | 3650.0 ± 115.8 | P1: <0.001 |
| P2: <0.001 | ||||
| P3: 0.091 | ||||
| CD4+ | 275.2 ± 41.2 | 638.5 ± 60.9 | 1057 ± 81.8 | P1:<0.001 |
| P2:<0.001 | ||||
| P3:<0.001 | ||||
| Th17 | 3.2 ± 0.24 | 2.53 ± 0.21 | 0.57 ± 0.13 | P1:<0.001 |
| P2:<0.001 | ||||
| P3: <0.001 | ||||
| Treg | 0.5 ± 0.14 | 1.0 ± 0.19 | 3.02 ± 0.17 | P1:<0.001 |
| P2:<0.001 | ||||
| P3:<0.001 | ||||
| T h17/Treg ratio | 3.15 ± 1.01 | 0.35 ± 0.13 | 0.24 ± 0.08 | P1:<0.001 |
| P2:<0.001 | ||||
| P3:<0.001 |
Table 4. Comparison between lymphocytes, CD4+, Th17, Treg and Treg/Th17 among active and inactive juvenile SLE patients and control, Note the ratio Th17/Treg in the above table was calculated case by case (for each case individually), then the mean was calculated for the calculated ratios.
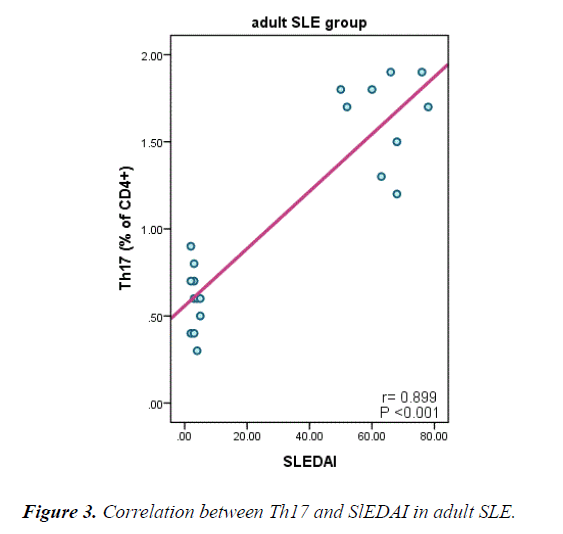
Table 5 and Figure 3 explain a positive correlation between Th17 in adult and juvenile SLE and duration of the disease, SLEDI, serum creatinine, ANA, Anti ds-DNA and, a negative correlation between Th17 and absolute neutrophils count, platelet C3 and C4.
| Variables | Adult SLE No=40 | Juvenile SLE No=40 | ||
|---|---|---|---|---|
| r. | p. | r. | p. | |
| Disease Duration | 0.73 | <0.001 | 0.685 | 0.001 |
| SLEDAI | 0.899 | <0.001 | 0.892 | <0.001 |
| Neutrophils count | -0.621 | 0.001 | -0.53 | <0.002 |
| Platelet count | -0.874 | <0.001 | -0.79 | <0.001 |
| Serum creatinine | 0.741 | <0.001 | 0.63 | 0.001 |
| Anti-ds-DNA | 0.928 | <0.001 | 0.934 | <0.001 |
| ANA | 0.933 | <0.001 | 0.93 | <0.001 |
| C3 | -0.928 | <0.001 | -0.92 | <0.001 |
| C4 | -0.896 | <0.001 | -0.93 | <0.001 |
Table 5. Correlation between Th17 and disease parameters.
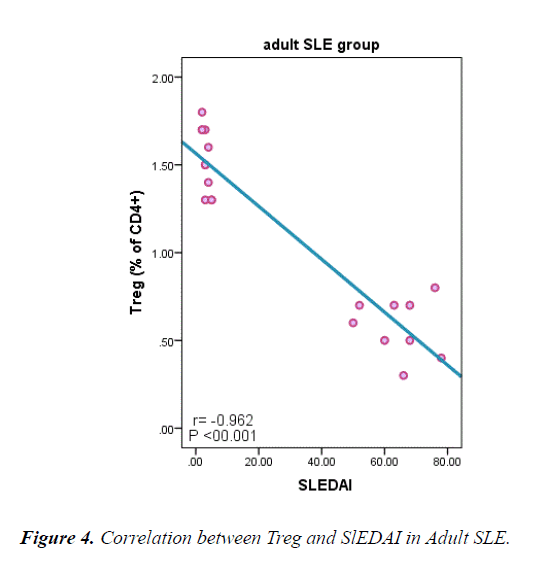
Table 6 and Figure 4 explain negative correlation between Treg cells in adult and juvenile SLE and duration of the disease, SLEDI, serum creatinine, ANA, and Anti ds-DNA and, a positive correlation between Treg cells and neutrophils count, platelet, C3 and C4.
| Variables | Adult SLE No.=40 | Juvenile SLE No.=40 | ||
|---|---|---|---|---|
| r. | p. | r. | p. | |
| Disease Duration | -0.658 | 0.002 | -0.609 | 0.004 |
| SLEDAI | -0.962 | <0.001 | -0.918 | <0.001 |
| Neutrophils count | 0.654 | 0.002 | 0.644 | 0.002 |
| Platelet count | 0.823 | <0.001 | 0.919 | <0.001 |
| Serum creatinine | -0.847 | <0.001 | -0.683 | 0.002 |
| ANA | -0.93 | <0.001 | -0.95 | <0.001 |
| Anti-ds-DNA | -0.944 | <0.001 | -0.944 | <0.001 |
| C3 | 0.923 | <0.001 | 0.919 | <0.001 |
| C4 | 0.927 | <0.001 | 0.943 | <0.001 |
| ANA | -0.93 | <0.001 | -0.95 | <0.001 |
Table 6. Correlation between Treg and disease parameters.
Discussion
Systemic lupus erythematosus (SLE) is an autoimmune disorder affecting almost all organs and tissues of the body. T-helper 17 (Th17) cells are differentiated from native CD4+T and are characterized by the production of IL-17 and have been included in a list of autoimmune diseases such as SLE as they are responsible for the inflammatory response in these diseases [17].
The other face of the coin in the pathogenesis of SLE is Treg cells which are a subset of CD4 T cells whose functions is to suppress the immune response and maintain self-tolerance [18]. The etiology and pathogenesis of SLE disease are still unclear; however, it has been shown that the balance between Th17 and Treg cells is a paradigm which underlies the progression of clinical symptoms in SLE patients [19].
The balance between Th17 and Treg in SLE patients has been studied for many years and giving different results, this may be due to disease heterogeneity, the impact of immunosuppressive treatment or these studies included patients with different levels of disease activity.
Most studies about Th17 and Treg which studied their relation to (SLE) disease showed that the elevation of Th17 and reduction of Treg were thought to be the major factor of increasing production of autoantibodies and tissue damage in SLE patients [20,21]. Our study showed a significant difference in ANA, DNA, and protein/creatinine ratio between adults, juvenile groups and controls.
Our study showed a significant increase in absolute number and percentage of Th17 cells and a significant decrease in absolute number and percentage of Treg cells with increased Th17/Treg ratio in active adult and juvenile SLE when compared with inactive SLE and healthy control. On comparing between inactive SLE and healthy control, there was no significant difference [22]. Our results were in agreement Xing and Talaat et al. and Ning et al. [23-25] and increased Th17 cell percentage was also observed in adult patients with active SLE. However, we disagree with Weronika Klecynska et al. [26] who did not find any difference in Th17 cells percentage between active SLE patients and healthy control.
Treg cells in SLE patients have been studied in many studies but the results obtained still confusing in which, most studies showed a reduced number of Treg [21] and other studies showed that instead of a reduction of Treg, the percentage of Treg increased in active SLE patients [27,28]. However, these Treg cells in SLE patients were found to have impaired suppressive function to control the autoimmunity process in SLE disease [29], or the pro-inflammatory Th17 cells are resistant to suppression action of regulatory T cells. Due to the difference of the value of Th17 and Treg cells in patients with active SLE from one report to another constantly the ratio of TH17/Treg will differ. Some studies showed an increase in Th17/Treg ratio in adult active SLE when compared with inactive and control [30].
Another research group described an elevated ratio of Th17 to Treg in adult patients with active SLE with no evidence of an increase in the percentage of Th17 [31]. Also, Yang et al. showed a significant increase of Th17/Treg ratio in SLE patients which indicate that Treg is incapable of modulating Th17 differentiation [32]. The imbalance of Th17/Treg ratio toward the side of pro-inflammatory Th17 side has been found in many of autoimmune diseases such as rheumatoid arthritis (RA), multiple sclerosis (MS), inflammatory bowel disease (IBD), as well as systemic lupus erythematous (SLE) [33].
The study also indicated a significant correlation between TH17/Treg ratio and parameters of the disease activity. Positive correlation with ANA, ANT-DNA, protein/creatinine ratio and SLEDAI with negative correlation with C3 and C4. These results are in agreement with those observed by Xing, Talaat et al. and Ning et al. [23-25]. Circulating number of Treg is decreased in SLE with unknown reasons. Environmental factors such as ultraviolet light infection and cigarette smoking cause oxidative stress and increased production of IL-6 which may be the primary cause for a decrease in Treg in SLE [34,35].
Ultraviolet exposure can induce DNA damage generation of DNA photoproducts and apoptotic materials with activation of B cells and promote the differentiation of Th17 cells with production of IL-17 and aggravate lupus injury as IL-17 induces an inflammatory response [34,35]. Normally TH17/ Treg ratio stays in a dynamic immune balance Increased oxidative stress in SLE induce and expand the pro-inflammatory Th17 cells and inhibit the anti-inflammatory Treg cell differentiation and aggravate autoimmune injuries [34,35].
Many drugs which capable of modifying Th17/ Treg ratio have been shown to be effective in the autoimmune diseases and approved for treatment of some of autoimmune diseases [36,37]. Also, it was found that the Th17 cells are not uniform subset but consist of pathogenic and non-pathogenic cell population. The non-pathogenic Th17 cells has a physiologic role in the protection of intestinal barrier function [38]. The therapeutic strategies which depend on Targeting only pathogenic Th17 cells may be the next step for shifting Th17/ Treg ratio. Recently, several molecules which capable of modifying the Th17/Treg imbalance have shown promising results in animal models [39,40]. But they need more evaluation before tested in human.
Conclusion
In summary, several challenges are still required identifying the Th17/Treg targeted therapeutic strategy that will be effective for the treatment of autoimmune diseases as well as SLE disease. In conclusion, elevation in pro-inflammatory Th17 and reduction of Treg is highly correlated to SLE disease activity in both adult and juvenile SLE patients. Returning Th17/Treg cell balance may open new lines for SLE treatment.
Further studies about Th17/Treg imbalance are required at several levels of disease activity because most studies include patients of low activity level and early diagnosed SLE patients in which reports that included both adult and juvenile SLE patients are not found.
References
- Crow MK. Etiology and pathogenesis of systemic lupus erythematosus. Elsevier, Philadelphia. 2017: 1329-1344
- Saeed M. lupus pathobiology based on genomics. Immunogenetices. 2017; 69: 1-12
- Agmon-Levin N, Mosca M, Petri M, et al. Systemic lupus erythematous one disease or many? Autoimmun Rev. 2012; 11: 593–595.
- C Scheinecker, M Bonelli, JS Smolen. Pathogenetic aspects of systemic lupus erythematosus with an emphasis on regulatory T cells. Journal of Autoimmunity. 2010; 35: 269–275.
- Alunno A, Bartoloni E, Bistoni O, et al. Balance between regulatory T and Th17 cells in systemic lupus erythematosus: the old and the new. Clin Dev Immunol 2012.
- Sakaguchi S, Miyara M, Costantino CM, et al. FOXP3+ regulatory T cells in the human immune system. Nature Reviews Immunology. 2010; 10: 490-500
- Buckner JH. Mechanisms of impaired regulation by CD4+CD25+ FOXP3+ regulatory T cells in human autoimmune diseases. Nature Reviews Immunology. 2010; 10: 849–859.
- Campbell DJ, Koch MA. Phonotypical and functional specialization of FOXP3+ regulatory T cells. Nature Reviews Immunology. 2011; 11: 119-130.
- Pons-Estel P. The American College of Rheumatology and the Systemic Lupus International Collaborating Clinics Classification criteria for systemic lupus erythematous in two multi-ethnic cohorts: A commentary. Lupus. 2014; 23: 3.
- Sag D. Performance of the new SLICC classification criteria in childhood systemic lupus erythematous: A multicentre study. Clin Exp Rheumatol. 2014; 32: 440-444.
- Touma Z, Gladman DD, Su J, et al. SLE Disease Activity Index Glucocortico-steroid Index (SLEDAI-2KG) Identifies More Responders Than Sledai-2K. Arthritis Rheumatol. 2017; 69.
- Castro C, Gourley M. Diagnostic testing and interpretation of tests for autoimmunity. J Allergy Clin Immunology. 2010; 125: S238-S247.
- Antico A, Platzgummer S, Bassetti D, et al. Diagnosing systemic lupus erythematosus:new-genration immunoassay for measurement of anti-dsDNA antibodies are effective alternative to the far technique and the crithidia luciliae immunofluorescence test. Lupus. 2010; 19: 906-912.
- Ritchie RF, Palomaki GE, Neveux LM, et al. Reference distributions for complement proteins C3 and C4; A practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 2004; 18: 1-8.
- Carvalho S. LiZIP3 is a cellular zinc transporter that mediates the tightly regulated import of zinc in Leishmania infantum parasites. Mol Microbiol. 2015; 96: 581-595
- Wang J, Jiang R, Peng S, et al. Immunologic control of Mus musculus papillomavirus Type 1. PLOS. 2015.
- Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T-cells regulates tissue inflammation by producing interleukin 17. Nature Immunology. 2005; 6: 1133–1141.
- Ma J, Yu J, Tao X, et al. The imbalance between regulatory and IL-17-secreting CD4+ T cells in lupus patients. Clin Rheumatol. 2011; 29: 1251-1258.
- Yang J, Yang X, Zou H, et al. Recovery of the immune balance between Th17 and regulatory T-cells as a treatment for systemic lupus erythematous. Rheumatology. 2011; 50: 1366-1372.
- Hang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009; 183: 3160-3169.
- Yang J, Chu Y, Yang X, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009; 60: 1472-1483.
- Zmyrka-Kaczmarek M, Kosmaczewska A, Ciszak L, et al. Peripheral blood Th17/Treg imbalance in patients with low-active systemic lupus erythematosus. Postepy Hig Med Dosw 2014; 68: 893-898.
- Xing Q, Wang B, Su H, et al. Elevated Th17 cells are accompanied by FoxP3+Treg cells decrease in patients with lupus nephritis. Rheumatol Int. 2011.
- Talaat RM, Mohamed SF, Bassyouni IH, et al. Th1/Th2/Th17/Treg cytokine imbalance in systemic lupus erythematous (SLE) patients: Correlation with disease activity. Cytokine. 2015; 72: 146-153.
- An N, Chen Y, Wang C, et al. Chloroquine Autophagic Inhibition Rebalance Th17/Treg-Mediated Immunity and Ameliorates systemic lupus erythematous. Cellular physiology and biochemistry. 2017; 44: 412:422.
- Kleczynska W, Jakiela B, lutecka H, et al. Imbalance between Th17 and regulatory T-cells in systemic lupus erythematosus. 2011; 49: 646-653.
- Handono K, Firdausi SN, Pratama MZ, et al. Vitamin A improve Th17 and Treg regulation in systemic lupus erythematous. Clinical rheumatology. 2016; 35: 631- 638.
- Venigalla RK, Tretter T, Krienke S, et al. Reduced CD4+, CD25 Tcell sensitivity to the suppressive function of CD4+, CD25high, CD127-/low regulatory T cells in patients with active systemic lupus erythematosus. Arthritis Rheum. 2008; 58: 2120–2130.
- Anaya JM, Ramirez-Santana. The Autoimmune Ecology. Front Immunol. 2016; 7: 139.
- Ma J, Yu J, Tao X, et al. The imbalance between regulatory and IL -17-secreting CD4+T cells in lupus patients. Clin Rheumatol. 2010; 29: 1251-1258.
- Han L, Yang, J, Wang X, et al. Th17 cells in autoimmune diseases. Front Med. 2015; 9: 10-19.
- Yang J, Yang X, Zou H, et al. Recovery of the immune balance between Th17 and regulatory T cells as a treatment for systemic lupus erythematosus. Rheumatology. 2011; 50: 1366-1372.
- Blauvelt A, Prinz JC, Gottlieb AB, et al. Administration by pre-filled syringe: Efficacy, safety and usability results from a randomized controlled trial in psoriasis (FEATURE). Br J Dermatol. 2015; 172: 484-493.
- Yang J, Chu Y, Yang X, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009; 60: 1472-1483.
- Shah K, Lee WW, Lee SH, et al. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res Ther. 2010; 12: R53
- Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015; 36: 422-439.
- Teitsma XM, Marijnissen AKA, Bijlsma JWJ, et al. Tocilizumab as monotherapy or combination therapy for treating active rheumatoid arthritis: A meta-analysis of efficacy and safety reported in randomized controlled trials. Arthritis Res Ther. 2016; 18: 211.
- Wang C, Yosef N, Gaublomme J, et al. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell. 2015; 163: 1413-1427.
- Baek SY, Lee JJ, Lee DG, et al. Ursolic acid ameliorates autoimmune arthritis via suppression of Th17 and B cell differentiation. Acta Pharmacol Sin. 2014; 35: 1-11.
- Takaishi M, Ishizaki M, Suzuki K, et al. Oral administration of a novel RORt antagonist attenuates psoriasis-like skin lesion of two independent mouse models through neutralization of IL-17. J Dermatol Sci. 2016.