Review Article - Journal of Medical Oncology and Therapeutics (2016) Volume 1, Issue 1
Stromal Fibrosis: A Complex Entity in the Day to Day on Breast Radiologist
Estela Fernandez Cuadriello*
Department of Radiology, Begona Hospital, Pablo Iglesias, Gijón, Asturias, Spain.
- *Corresponding Author:
- Estela Fernandez Cuadriello
Department of Radiology, Begona Hospital,
Pablo Iglesias, Gijón, Asturias,
Spain.
E-mail: estelacuadriello@gmail.com
Accepted date August 08, 2016
DOI: 10.35841/medical-oncology.1.1.19-23
Visit for more related articles at Journal of Medical Oncology and TherapeuticsAbstract
I want to describe an increasingly common condition in our hospital and radiological interest given its diagnostic difficulty in benign breast disease, both from the point of view mammographic and sonographic and anatomic-pathological correlation. So we've made a brief retrospective review of cases in our hospital in the last 22 months, with the aim of trying to establish a profile you provide radiological diagnosis of Stromal Fibrosis
Keywords
Stromal fibrosis, Breast, Radiologist, Review, Diagnosis.
Introduction
A large part of both clinical and subclinical lesions found in our daily work are benign. Part exhibit traits that define characteristically in different techniques applied and diagnose correctly, there is another small group of processes that can take the aspect image of a cancer, exhibiting an irregular morphology or indistinct borders, predominantly vertical diameter on the horizontally or associating intense shadows, among others.
Focal fibrosis of breast is a benign condition, which is characterized as obliterated acinar and ductal elements with hypocellular fibrous tissue on histopathological examination. Focal fibrous stroma also surrounds atrophic epithelium; however, none of these histopathological findings are specific. The entity may occur in patients without any clinical or radiological findings [1].
Stromal fibrosis is known for multiple terms: "fibrous mastopathy", "breast fibrosis," "fibrous breast disease," "fibrous tumor" breast and "focal fibrosis" [2,3]. The diagnosis has become increasingly common and may represent as much as 10% of lesions found in patients who undergo imagingguided core biopsy [3-6].
It is histologically described as a proliferation of intra and interlobular connective tissue which becomes progressively denser, compromising the epithelial set (ductal), which partly comes to disappear and does in many cases not possible to recognize the lobules as such. In the first moments a lymphoid infiltrate the stroma Intralobular seen, arriving in advanced to the latter adopts an almost hyaline aspect phases. Many authors confer the title of involution feature of the breast, rather than an actual entity histopathologic said.
Today some doubts about its origin still arise, on the one hand it is thought that there might be an estrogen-dependent factor underlying fibroblast proliferation, but without epithelial effect, which could be supported by the fact that in this study as in other series, is predominant in premenopausal women, on the other hand is postulated that it is a variant in the glandular involution process, there is even the point in question to the end of a previous inflammatory process. It was initially reported as a palpable breast mass in premenopausal women or postmenopausal women receiving hormone replacement therapy [7]. However, it has become an increasingly common diagnosis after core needle biopsy of clinically occult imaging-detected abnormalities [3-7].
Venta and cols established a classification of stromal fibrosis based on the different patterns [5]:
• Type I or perilobular fibrosis: is fibrosis of perilobular elastic connective tissue with expansion and enlargement of ductolobular units. The perilobular collagen rings may form small nodules. Revelon et al. [6] described this entity as “nodular fibrosis.”
• Type 2 or septal fibrosis, is fibrosis involving the interlobular stroma, leading to widening of the preexisting septal collagen bands.
• Type 3 or haphazard fibrosis, is interlobular fibrosis resembling a scar, with thick fibrotic bands extending peripherally in a random to radial manner from a central focus, associated with architectural distortion. This type is frequently seen with fat necrosis and radial scar.
In their serie, they observed that these patterns of fibrosis might be present in a single leison, but in a high porcentaje were mixed, one dominant pattern and other minor.
The imaging features reported include benign-appearing masses as well as lesions that can simulate malignancy [2-6]. With the advent of contrast-enhanced breast MRI and MRI-guided core biopsy, stromal fibrosis has emerged as a common false-positive diagnosis on breast MRI [8]. Although it is well documented that stromal fibrosis can have variable appearances on mammograms and sonograms, sometimes mimicking malignancy [3-6] only a few MRI scans of stromal fibrosis appear sporadically in the literature [8,9].
In our case series it is a 32%, 28 out of 87 bening biopsies, all ultrasound-guided, with a mean age of patients 48.8 years and being slightly predominant premenopausal. All of them were subclinical and in 3 patients histopathologic findings associated a component of sclerosing adenosis and in 3 cases unspecific chronic inflammatory changes.
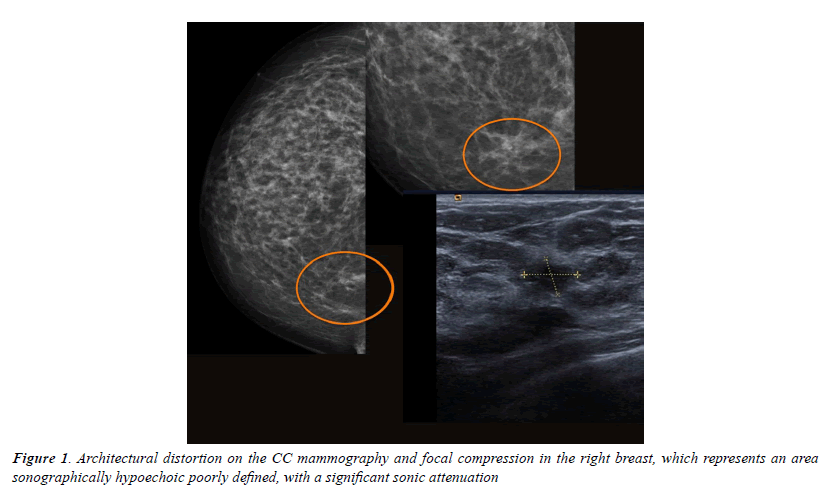
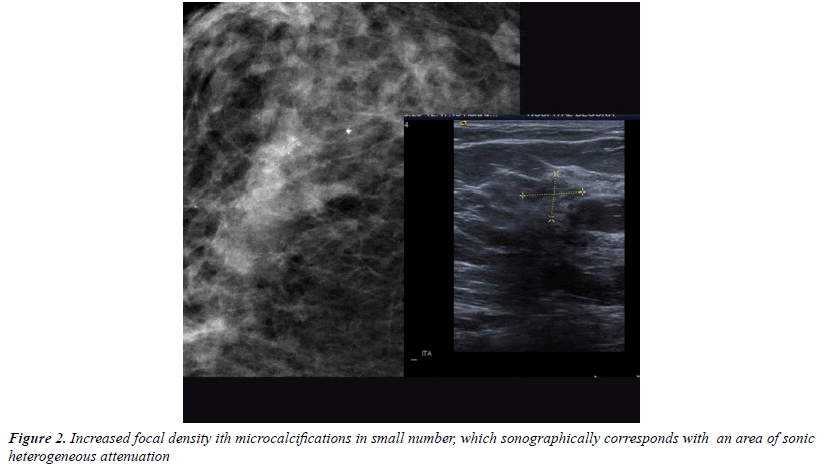
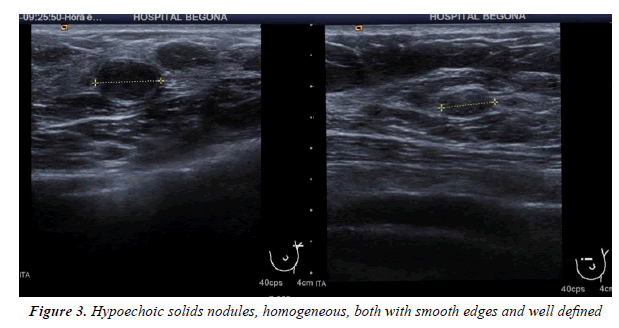
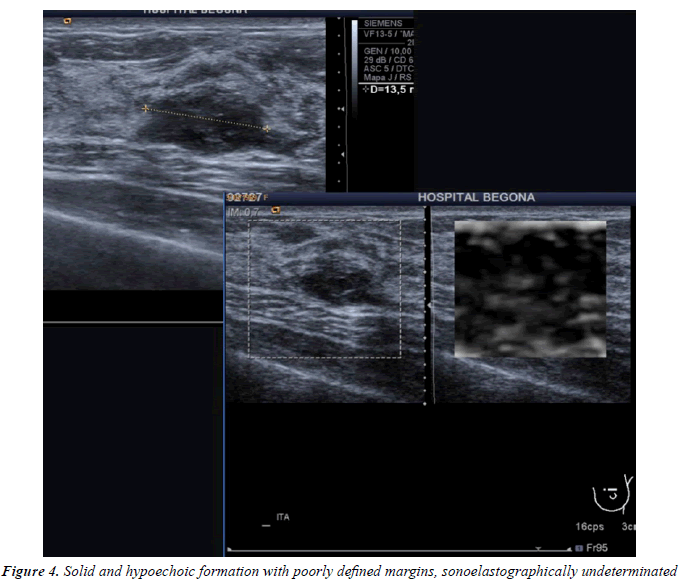
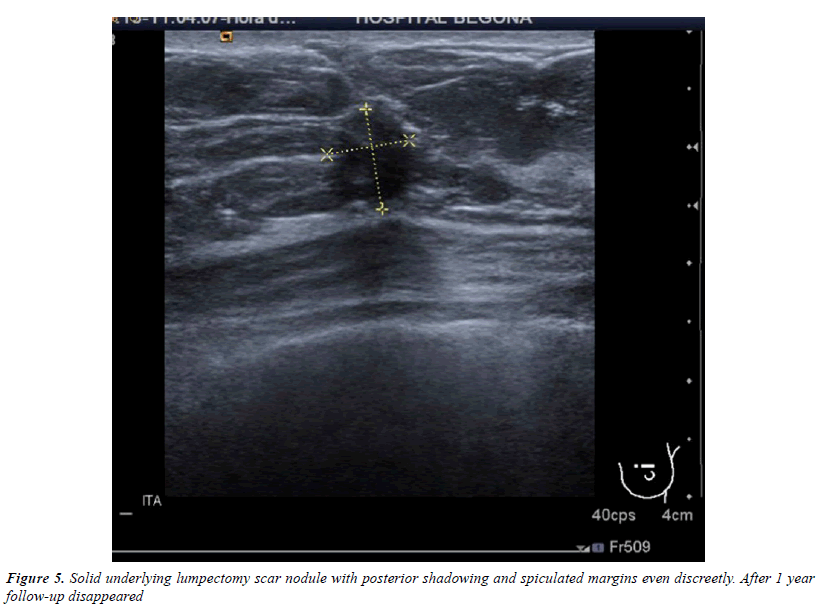
All injuries the have characterized in ultrasound, identifying only 6 in the previous mammography (although in 3 cases mammography was done outside our center and we did not have it): 3 represented by an area of architectural distortion (Figure 1), 1 as a nodule/mass, one focal asymmetry and one cluster of microcalcifications (Figure 2). On sonography, however, most have corresponded with nodules (19 cases), respecto 9 areas of sonic attenuation shadowing. From these seven nodules showed smooth and well defined margins (Figure 3), 7 lobed, 2 microlobulated (Figure 4) and five fuzzy edges and slightly irregular (Figure 5), considering that the echostructure was in practically all cases hypoechoic, only three resulted heterogeneous and slightly hyperechogenic.
The most common site was the CSE, a priori very possibly because it is the area with the greatest amount of breast tissue and second UCS-CSI, like the Revelon´s study. The average size lesional was 14,5 mm. Several hypotheses have been proposed to explain the fibrotic nodule formation. One of them is the hormonal stimulation of fibroelastic tissue without any stimulus on mammarian epithelial cells [2-4]. Previous studies also state that focal fibrosis is present more often in pre-menopausal women.
Before the era of screening mammography, focal breast fibrosis was diagnosed infrequently and the mammographic and sonographic appereance are nonspecific and variable [2,3,5,6]. Recent studies reported that many of them were presented as well-defined benign-looking masses on a sonographic examination, but sonographic evaluation using the Breast Imaging Reporting and Data System (BI-RADS) has not been reported [10]. A part of the patients may present well-circunscribed benign-appearing masses and are suitable for a follow-up protocol [2,11,12], but stromal fibrosas is an entity which simulates frequently malignancy.
In the diagnosis of palpable masses or lesions detected on radiological evaluation, imaging guided core needle biopsy of the breast is a widely used procedure. The procedure provides reliable histopathological results with a cost-effective and minimal invasive way [13-15].
Radiologic-pathologic concordance is important to establish, specially for noncalcified lesions, to minimize the risk of a delayed diagnosis of breast cancer. Subsequent to USguided or stereotactic core biopsy, upon receipt of pathology, the board certified radiologist who performed the biopsy must review the pathology reports in conjunction with the mammographic and/or US images to determine concordance. Pathology is determined to be concordant when the reported findings provide an acceptable explanation for the imaging features. In cases where the histologic results are not sufficient to explain the imaging findings, the results were deemed discordant [16].
The false-negative rate (the number of cancers “missed” initially because of sampling error) is difficult to stablish from the literature because the follow-up has been limited [2]. It is generally accepted that a woman who has undergone breast biopsy with benign pathology is at increased risk for future development of breast cancer [16]. Although there is no generally accepted consensus on the management of stromal fibrosis, it has been suggested that the histopathology diagnosis of stromal fibrosis should be considered concordant with a benign diagnosis during radiology–pathology correlation, if accurate targeting is confirmed and in the absence of imaging features that are concerning for malignancy. However, follow-up imaging protocols after concordant benign breast biopsy vary by institution and no standard follow-up imaging guidelines for concordant benign lesions have been established [17,18]. The 2010 and 2013 consensus guidelines published by the National Comprehensive Cancer Network (NCCN) recommend follow-up diagnostic imaging and physical exam every 6-12 months for 1-2 years following a concordant benign core needle biopsy, prior to releasing these women back into the general screening population [19].
Core needle biopsy may be regarded as a sufficient and safe way for the management of such patients, especially if the radiological findings suggest a probably benign nature. However, if any suspicious finding is present in radiological work-up, follow-up or biopsy options should be decided by multi-disciplinary approach. Followup depends on patient’s coordination, so re-biopsy or surgical excision should be the first option in potentially uncoordinated cases [1].
Conclusion
Stromal fibrosis is a complex entity that is increasingly more often mainly due to increased number of biopsies we perform today and has a wide spectrum that often mimics breast cancer. We believe that the breast radiologist should have this feature very present, whose diagnosis is a major challenge in sharing features of malignancy and multiple forms of manifestation, which makes the clasification many as BIRADS 4. Therefore, we believe appropriate and necessary histological characterization of this entity in all cases until we acquire a greater degree of experience, having to be extremely rigorous as regards the histopato- radiological agreement. Also, I consider necessary control of these patients, and it would be very interesting to investigate in appropriate patterns of short-term monitoring.
References
- Taskin F, Unsal A, Ozbas S, et al. Fibrotic lesions of the breast: Radiological findings and core-needle biopsy results. Eur J Radiol 2011; 80: e23-236.
- Sklair-LM, Samuels TH, Catzavelos C, et al. Stromal fibrosis of the breast. AJR 2001; 177: 573-577.
- Harvey SC, Denison CM, Lester SC, et al. Fribous nodules found at large-core needle biopsy of the breast: imaging features. Radiology 1999; 211: 535-540.
- Rosen EL, Soo MS, Bentley RC. Focal fibrosis: A common breast lesion diagnosed at imaging-guided core biopsy. AJR 1999; 173: 1657-1662.
- Venta LA, Wiley EL, Gabriel H, et al. Imaging features of focal breast fibrosis: Mammographic-pathologic correlation of non-calcified breast lesIons. AJR 1999; 173: 309-316.
- Revelon G, Sherman ME, Gatewood OMB, et al. Focal fibrosis of the breast: Imaging characteristics and histopathologic correlation. Radiology 2000; 216: 255-259.
- Rosen PP. Benign mesenchymal neoplasms. Rosen´s breast pathology, 3rd ed. Philadelphia, PA: Lippincot Williams & Wilkins 2009: 838-839.
- Lee SJ, Mahoney MC, Khan S. MRI features of stromal fibrosis of the breast with histopathologic correlation. AJR 2011; 197: 755-762.
- Gutierrez RL, DeMartini WB, Eby PR. BIRADS lesion characteristics predict likelihood of malignancy in breast MRI for masses but not for nonmass like enhancement. AJR 2009; 193: 994-1000.
- You JK, Kim EK, Kwak JY, et al. Focal fibrosis of the breast diagnosed by a sonographically guided core biopsy of nonpalpable lesions. J Ultrasoun Med 2005; 24: 1377-1384.
- Guenin M. The low false-negative rate for stereotactic breast biopsy (letter). Radiology 2000; 216: 609-610.
- Lee CH. Follow-up of breast lesions diagnosed as benign with stereotacytic core-needle biopsY. Radiology 1999; 212: 189-184.
- Liberman L, Ernberg LA, Heerdt A, et al. Palpable breast masses: Is there a role for percutaneous imaging-guided core biopsy? Am J Roentgenol 2000; 175: 779-787.
- Berg WA, Hruban RH, Kumar D, et al. Lesions from mamographic-histopathologic correlation of a large-core needle breast biopsy. Radiographics 1996; 16: 1111-1130.
- Liberman L. Percutaneous imaging-guided core breast biopsy: State of the art at the millenium. AJR 2000; 174: 1191-1199.
- Adams M, Falcon S, Mooney B, et al. Short-term imaging follow-up of patients with concordant benign breast core needle biopsies: Is it really woth it? Diagn Interv Radiol 2014; 20: 464-469.
- March DE, Raslavicus A, Coughlin BF, et al. Use of breast core biopsy in the United States: Results of a national survey. AJR 1997; 169: 697-701.
- Youk JH, Jung I, Kim EK, et al. US follow-up protocol in corcondant benign result after US-guided 14-gauge core needle breast biopsy. Breast Cancer Res Treat 20012; 132: 1089-1097.
- Guidelines for breast cancer screening and diagnosis, NCCN Guidelines Version 2. 2013. National Comprehensive Cancer Network. 2013.