Review Article - Journal of Food Technology and Preservation (2022) Volume 6, Issue 6
Starch chemistry and application.
Akama Friday Ogori1*, Alimi Taofeek21Department of Home Sciences, Faculty of Agriculture, Federal University Gahua, 671106, Gashua, Nigeria
2Department of Chemistry, Federal College of Education, Kontagora, Niger State, Nigeria
- *Corresponding Author:
- Akama Friday Ogori
Department of Home Sciences
Faculty of Agriculture
Federal University Gahua
671106, Gashua, Nigeria
E-mail: ogorifaraday@gmail.com
Received: 25-Mar-2022, Manuscript No. AAFTP-22-63526; Editor assigned: 29-Mar-2022, PreQC No. AAFTP-22-63526(PQ); Reviewed: 13-Apr-2022, QC No. AAFTP-22-63526; Revised: 25-May-2022, Manuscript No. AAFTP-22-63526(R); Published: 02-Jun-2022, DOI:10.35841/2591-796X-6.6.130
Citation: Ogori AF. Starch chemistry and application. J Food Technol Pres. 2022;6(6):130
Abstract
The starch chemistry aspect of amylose and amylopectin fractions along with their contributory roles in defining starch food behavior; such as in gels formation, pasting quality, swell ability, dextrinization and viscosity from various sources such as legumes, root, tubers and cereals were reviewed. Chemical and physical modifications of starch like cationation, esterification, acetylation, alkalization and annealing, which somehow weakens glucose linked bond and releasing fractional starch moieties have shown to modify characteristic behavior of starch in food systems and for non- food systems. The application of starch in food and non-food systems were envisaged from resistant to non-resistance starches in legumes, roots and tubers and these were observed to be better in functional characteristic than cereal starches.
Keywords
Cereals, Food system, Legumes, Non-food systems.
Introduction
Fractions from carbohydrate called starch are very important in the human diet and accounts for a considerable percentage of our carbohydrate intake. A starch occurs in plants in the form of granules, and these are particularly abundant in seeds especially the cereal grains and tubers, where they serve as a storage form of carbohydrates [1]. It is used in a wide range of foods for a variety of purposes including thickening, gelling, adding stability and replacing or extending food systems [2]. Food starches are favored for their availability, comparatively low cost and unique properties; their native form is a versatile product, and the raw material for production of many modifications, sweeteners and ethanol [3]. Starch is isolated mainly from corn, potatoes, cassava, and wheat in the native and modified forms and this account for 99% of the world production [4]. Some other starches are also available commercially; recently, starches obtained from legumes (peas, lentils) have become more interesting because they have properties which appear to make them a suitable substitute for chemically modified starches for series of products [5]. This food source has various origins and individual, characteristic properties which go back to the shape, size, size distribution, composition, and crystallinity of the granules [3]. The process of obtaining starch varies; however, plant as source of material is disintegrable. In other cases, such as cereals, the starch is embedded in the endosperm protein matrix; hence granule isolation is a more demanding process. Thus, a counter-current process with water at 50°C for 36 to 48 hours is required to soften corn (steeping process). The steeping water with 0.2% SO2 loosens the protein matrix and, thereby accelerates the granule release and increase the starch yield [6].
Structure and properties of starch granules
Starch is a mixture of two polymers: amylose and amylopectin. Natural starches consist of about 10 to 30% amylose and 70 to 90% amylopectin [5].
Amylose: It is a linear polysaccharide composed entirely of D-glucose units joined by the α-1,4-glycosidic bonds. Evidence indicates that amylose is not a straight chain of glucose units but coiled like a spring, with six glucose monomers per turn. When coiled in this fashion; amylose has enough room in its core to accommodate an iodine molecule. The characteristic blue-violet color that appears when starch is treated with iodine is due to the formation of the amylose-iodine complex.
Amylopectin: It is a branched-chain polysaccharide composed of glucose units linked primarily by α-1,4- glycosidic bonds but with occasional α-1,6-glycosidic bonds, which are responsible for the branching. A molecule of amylopectin may contain many thousands of glucose units with branch points occurring about every 25 to 30 units.
Derivatives of Starch
Dextrin: Dextrines are glucose polysaccharides of intermediate size. This could be seen in the shine and stiffness imparted to clothing by starch when clothing is ironed. Dextrin is more easily digested than starch and are therefore used extensively in the commercial preparation of infant foods [3]. The complete hydrolysis of starch yields, in successive stages such as; starch → dextrins → maltose → glucose patern. However, in the human body, several enzymes known collectively as amylases degrade starch sequentially into usable glucose units [3].
Glycogen: Glycogen is the energy reserve carbohydrate of animals. Practically all mammalian cells contain some stored carbohydrates in the form of glycogen, but it is especially abundant in the liver (4 to 8% by weight of tissue) and in skeletal muscle cells (0.5 to 1.0%). Like starch in plants, glycogen is found as granules in liver and muscle cells. When fasting, animals draw on these glycogen reserves during the first day without food to obtain the glucose needed to maintain metabolic satisfaction and balance [7].
Cellulose: Like amylose, cellulose is a linear polymer of glucose. It differs, however, in that the glucose units are joined by β- 1,4-glycosidic linkages, producing a more extended structure than amylose. This extreme linearity allows a great deal of hydrogen bonding between hydroxyl groups on adjacent chains, causing them to pack closely into fibers. As a result, cellulose exhibits little interaction with water or any other solvent. Because cellulose does not have a helical structure, it does not bind to iodine to form a colored product. Cellulose yields D-glucose after complete acid hydrolysis [3].
Classification of Starch
Waxy corn starch: Waxy corn starch, also known as waxy maize starch, consists of only amylopectin molecules, giving these starch different and useful properties. The starch stains red with iodine, not blue as ordinary starches do. When the corn kernel is cut, the endosperm appears shiny and wax-like, and the corn termed waxy corn or waxy maize.
High-amylose starch: The term amylose composed only of amylose molecules. High-amylose starch is used primarily by candy manufacturers who utilize high strength gels to help give candy shape and integrity. Addition of modified high amylose starch can enhance the texture of foods such as tomato paste and apple sauce. The ability of amylose starches to form films led to widespread investigation of its use in industrial products, including degradable plastics
Types of resistance Starch
Class of resistance starch includes; slow digestible starch (SDS), physically inaccessible starch (PIS 1), resistant granule (RG) and retrograded starch (Rs3) [8]. During the processing of starchy foods, starch is affected by many factors such as water content, pH, heating temperature and time, number of heating and cooling cycles, freezing and drying and the presence of additives such as oil, spice powder and salt. Resistance starch formation also depends on the amylose/ amylopectin ratio, starch-protein interactions, amylose- lipid complexes and the rate of starch retrogradation [7].
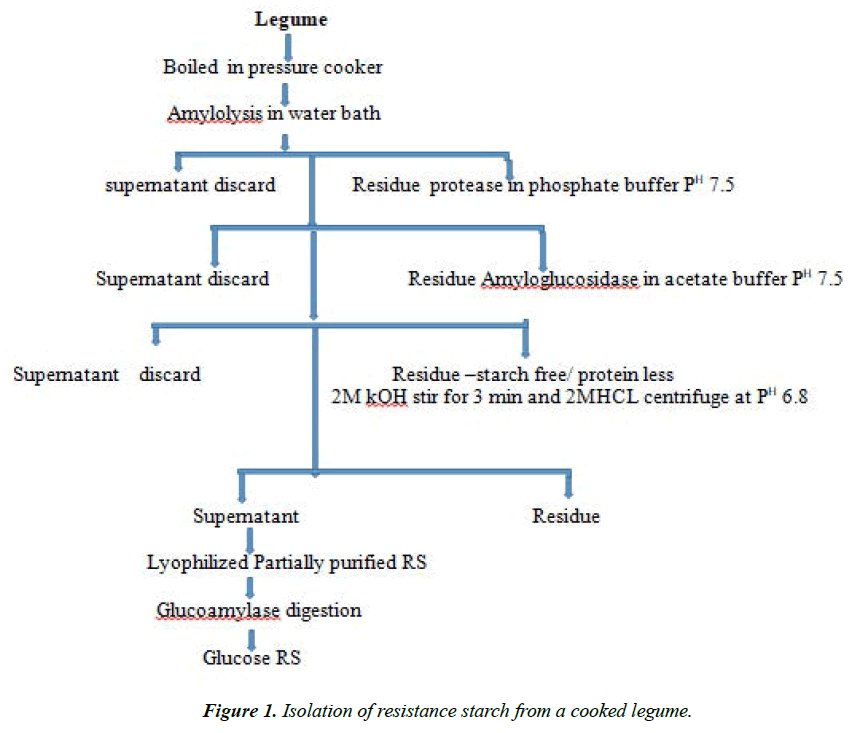
Resistance Starch from legumes
Autoclaved legume samples showed comparatively lower resistance starch values than pressure cooked samples (Figure 1) [9] showed that repeated autoclaving could increase the resistance starch content in cereals like ragi and rice. Germination is another traditional processing method commonly used, in which the whole grain is allowed to sprout. Fresh and sprouted legumes have been found to be rich sources of vitamin C and thiamin. The activities ofmany enzyme increases during controlled germination. The resistance starch content of the germinated grains compared with other processed materials decreased and the values ranging from 0.42 to 0.64%. Carbohydrates including starch are convertible into simpler molecules by the action of various hydrolyzing enzymes resulting in a diminished availability of native starch molecules for resistance starch.
Starch modification
The performance and quality of starch can be improved through chemical modification. Chemical modifications provides processed foods; such as frozen, instant, dehydrated, encapsulated and heat-and-serve products, the appropriate texture, quality and shelf life and also improved processing condition and tolerance, such as improved heat, shear and acid stability are also attributed to modification of starch [10]. Modification also allows starches to be used in the paper industry as wet-end additives, sizing agents, coating binders, and adhesives and as textile sizes. Carbohydrate Starches are inherently unsuitable for most applications and, therefore, must be modified chemically and/or physically to enhance their positive attributes to minimize their defects,these defects could be used in food products as thickeners, gelling agents and encapsulating agents, in papermaking as wet-end additives for dry strength, surface sizes and coating binders, as adhesives, envelopes, tube-winding and wallpaper pastes [11].
Various starch products such as thermoplastic starch and starch-polymer composites are used to replace petroleumbased plastics in some applications, newer applications include use of non-digestible starch as neutraceuticals,and a future of starch may include a role in detergents [12].
Modification of starch generally involves esterification, etherification or oxidation of the available hydroxyl groups on the α -D-glucopyranosyl units that make up the starch polymers. Similarly, another process involves the use of a cylindrical turbo-reactor for etherification, esterification and acid modification [13].
Chemical modification of Starch
Cationization: The degree of substitution (DS) of normal, waxy, high-amylose starches with 3-chloro-2-hydroxypropyltrimethylammonium chloride (cationizing reagent) in an alkaline alcoholic semi aqueous media for seven hours is termed cationization. Cationic starches are widely used as wetend additives in the pulp and paper industry. Cationic starches have significant use in papermaking as wet-end additives for dry Strength, as emulsion stabilizers for internal, synthetic sizing agents, such as alkyl ketene dimer and alkenyl succinic anhydride, and as surface sizing agents [2].
Modification by crosslinking
Cross-linked starches are obtained by the reaction of starch (R- OH) with bi- or polyfunctional reagents, such as sodium trimetaphosphate, phosphorus oxychloride, epichlorohydrin or mixed anhydrides of acetic and dicarboxylic acids The conditions for crosslinking of waxy barley starch is between pH 11.1-11.5 with phosphorous oxychloride (POCl3) at 0.005 – 0.007% and a reaction time of 20 – 60 minutes. At the same pH and reaction time, the optimum level of POCl3 to crosslink waxy maize starch is 0010 – 0.015% POCl3 [14]. Native starches are sensitive to high temperature, shear stress and acid treatment when Cooked in water [14]. Starches used in the food industry are modified by crosslinking in order to improve their functionality in foods that are subjected to high temperature processing and shear stress but crosslinking does not impart freeze – thaw stability [15].
2R-OH+R1CO-O-CO-(CH2)n-CO-O-COR1→R-O-CO- (CH2)n-CO-O-R(5)
Acetylation: Acetylation is achieved by reaction with acetic anhydride or vinyl acetate. The improved smoothness and sheen attained leads to canned soup and sauce processors to switch from using only native corn or potato starch, and popularized the more general use of modified waxy maize starch [16]. The improved freeze – thaw stability of acetylated cross linked waxy maize starch led to the marketing of frozen sauces, initially on vegetable.
Modification by substitution
The hydroxyl groups of starch can be reactive and substituted by a range of functional groups for modifications. Starches from several species have been subjected to carboxymethylation [17,18], hydroxypropylation [19,20], acetylation, and succinylation, in which all these processes increased the hydrophilic properties of starch [19].
Modification by alkali treatment
Starch could be subjected to alkali treatment (0.1% NaOH) at 35 °C up to 30 days. The recovery of starches ranged from 71% to 93%.Alkali treatment decreased the amylose content. The hydrolysis increased the water solubility, while decreasing the swelling power of starch. The alkali treatment either increased or decreased the RS content, depending on the reaction conditions and the starch type. The changes in the molecular structure of starch by alkaline treatment have not yet been studied [21].
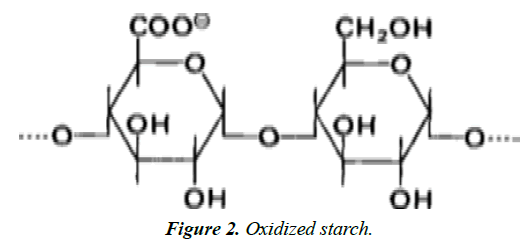
Oxidized modified Starches: Starch hydrolysis and oxidation occur when aqueous starch suspensions are treated with sodium hypochlorite at a temperature below the starch gelatinization temperature range. The products obtained have less of carboxyl group per 25–50 glucose residues. Oxidized starch is used as lower-viscosity filler for salad dressings and mayonnaise. Unlike thin boiling starch, oxidized starch does not retrograde nor does it set to an opaque gel [3] (Figure 2).
Thin-boiling starch: Partial acidic hydrolysis yields a starch product which is not very soluble in cold water but is readily soluble in boiling water. The solution has a lower viscosity than the untreated starch, and remains fluid after cooling. Retro gradation is slow; these starches are utilized as thickeners and as protective films.
Starch ethers: When a 30–40% starch suspension is reacted with ethylene oxide or propylene oxide in the presence of hydroxides of alkali and/or alkali earth metals (pH 11–13), hydroxyethyl- or hydroxypropyl derivates are obtained (R_ =H, CH3) [3].
The derivatives are also obtained in reaction with the corresponding epichlorohydrins. The substitution degree can be controlled over a wide range by adjusting process parameters. Low substitution products contain up to 0.1molealkyl group/ mole glucose, while those with high substitution degree have 0.8–1 mole/mole glucose.
Physical modification of starch
Extrusion: Pregelatinized starch of D. alata was produced by extrusion, which disrupted the structure [22]. The extruded starch had decreased retrogradation compared to native starch. It had high cold-thickening capacity and high gel strength, and it was recommended for instant creams and pudding manufacturing [22].
Heat-Moisture Treatment: Heat-moisture treatment (HMT) of starch refers to treating samples at moisture levels less than 35% for a certain period of time (up to 16 H). The temperature for HMT (84 to 120°C) is above the glass transition temperature and below the gelatinization temperature [23]. HMT of starches from D. hispida and D. alata decreased the swelling, solubility, and amylose leaching, while inducing the B-type polymorph in the granules to A-type [24]. HMT either increased or decreased the parameters of pasting and gelatinization processes.
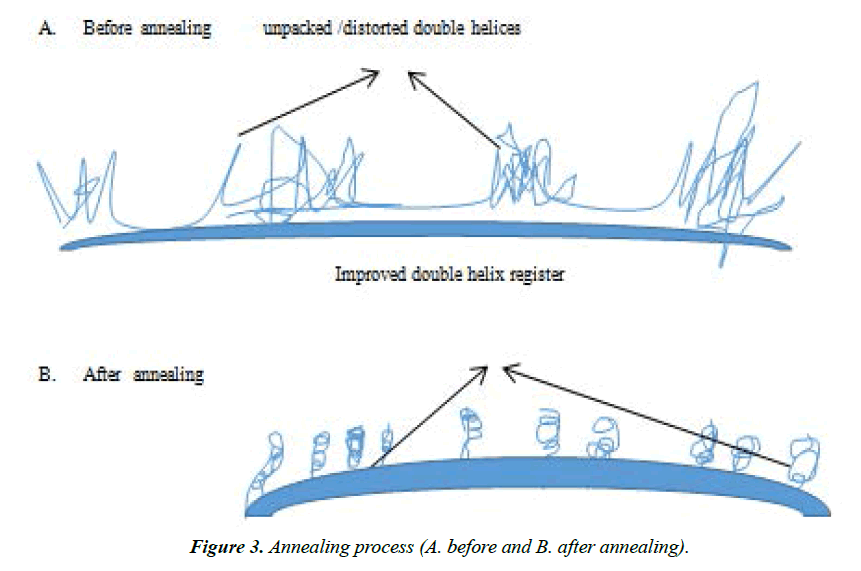
Annealing: Annealing is a process whereby a material is held at a temperature somewhat lower than its melting temperature, which permits modest molecular reorganization to occur and a more organized structure of lower free energy [19]. Studies have shown that changes to starch structure and properties occur on annealing. Recently, Waduge [25] showed that the relative crystallinity (RC) of waxy (zero-amylose, 7.8% amylose), normal (32 and 34% amylose) and highamylose (44 and 55% amylose) barley starches increased on annealing (at 50°C for 72 hours at a moisture content of 75%). However, RC of the high-amylose barley starches remained unchanged. The x-ray pattern remained unchanged on annealing in normal (32% amylose) and waxy starches, but changed from A- to an A _B pattern in normal (34% amylose) and high amylose starches.
Annealing increased the gelatinization transition temperature and decreased the gelatinization temperature range in all starches. The enthalpy of high-amylose (55% amylose) starches increases on annealing, whereas it remained unchanged in the other starches. In all starches, granular swelling decreased on annealing. Annealing decreased amylose leaching in normal (34% amylose) and high-amylose starches in the temperature range of 50 to 90°C, but increased amylose leaching in waxy (7.8% amylose) and normal (32% amylose) starches at higher temperatures. The Annealing patterns are as shown in Figure 3.
Mechanical modification
When starch granules are damaged by grinding or by application of pressure at various water contents, the amorphous portion is increased, resulting in improved dispersibility and swellability in cold water, a decrease in the gelatinization temperature by 5–10, and an increase in enzymatic vulnerability. In bread dough made from flour containing damaged starch, for instance, the uptake of water is faster and higher and amylose degradation greater.
Extruded starch: The X-ray diffraction diagram changes on extrusion of starch. The V-type appears first, followed by its conversion to an E-type at higher temperatures (>185°C), and reformation of the V-type on cooling. The E-type apparently differs from the V-type only in the spacing of the V helices of amylose [3]. Extruded starches are easily dispersible, better soluble, and have a lower viscosity. The partial degradation of appropriately heated amylose shows that chemical changes also occur at temperatures of 185–200°C. Apart from maltose, iso maltose, gentiobiose, sophorose, and 1,6-anhydroglucopyranose appeared.
Dextrinized starch: Heating of starch (<15% of water) to 100–200°C with small amounts of acidic or basic catalysts causes more or less extensive degradation. White and yellow powders are obtained which deliver clear or turbid, highly sticky solutions of varying viscosity. These products are used as adhesives in sweets and as fat substitutes.
Pregelatinized starch: Heating of starch suspensions, followed by drying, provides products that are swell- able in cold water and form pastes or gels on heating. These products are used in instant foods, for example, pudding, and as baking aids.
Sources and quality of Starch
Some properties of starches from cassava, potato and sweet potato were compared with cereal starches from maize, wheat, millet and sorghum [26,27]. Significant variations were observed for amylose content and solution properties of starches, where blue values for amylose ranged from 0.355 in potato to 0.476 in cassava, but were averagely low in cereal starches. Amylose leaching increased with temperature with the highest value in cassava at 80°C compared with cereal starches [27,28]. Starch amylose increased with time of hydrolysis and was highest for millet and sorghum and least for potato. According to [26], average swelling power at 80°C was high for cassava and potato (8.44 g/g) compared with sweet potato (6.88 g/g) and low among cereal starches (5.17 g/g). Similarly, starch solubility was low in potato (0.77 g/g) and sweet potato compared with cassava. The paste clarity was also high for cassava (48.32%) and potato (42.16%) and least for sweet potato derived starches (23.22%) and all the cereal starches (14.97%). These properties demonstrate the untapped potential of cassava and tuber based starches for use in food and non-food applications previously dominated by cereal starches; considerable variations occur in starch [26], amylose properties of starches from different botanical sources. It was also observed that though there might be similarities in the starches from different botanical sources, these similarities are variety specific making recommendation of crop specific applications difficult. As opined that different crops can be put to the same application making selection and use of cheap available sources for starch possible [27]. According to [27], the possibility of applying tuber and root crop starches for purposes that were once tagged to cereal starches. In fact, in some instances, tuber and root crop starches exhibited superiority over cereal starches (Table 1)
| Property | Wheat starch | Potato starch | ||
|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |
| Start of gelatinization(°C) | 56.5 | 61 | 60 | 60.5 |
| Water binding capacity (%) | 89.1 | 182.6 | 102 | 108.7 |
| End of gelatinization (°C) | 62 | 74 | 68 | 79 |
| Swelling capacity at 80°C (ratio) | 7.15 | 5.94 | 62.3 | 19.05 |
| Solubility at 80°C (%) | 2.59 | 5.93 | 31 | 10.1 |
Table 1. Physicochemical properties of starches before (1) and after (2) Heat Treatment in the Wet State (T = 100°C, t = 16 h, H2O = 27%).
Products from starch
Sweeteners: Starch hydrolysis led eventually to today’s modern starch sweetener industry. The original starch-derived sweeteners are mainly linear chains of starch. Before this, little was known about the structure or identity of starch polymers. The hydrolysis could start with a suitable starch to yield glucose or glucose syrup which when additives are employed forms sweetening substance [3].
Ethanol: Glucose syrups are easily fermented by yeast to ethanol. While beverage ethanol has been produced from many sources of sugar and starch for countless centuries, large scale production of fuel-grade ethanol by fermentation is attributed to a demand for combustible motor fuel additives. Today, most ethanol is made from corn starch. After separation from corn by wet milling, starch slurry is thinned with alpha-amylase with amyloglucosidase. The resulting sugar solution is formed which offers the advantages over batch fermentation of lower capital cost for fermenters, improved microbiological control, and ease of automating control of the process.
Polyols: Hydrogenation of sugars produces a class of materials known as sugar alcohols or polyols. Major commercial sugar alcohols include Manitol, sorbitol (D-glucitol), malitol, and xylitol and syrups related to these products, with all but xylitol being obtainable from starch by hydrolysis, isomerization in the case of mannitol, and hydrogenation. Sugar alcohols are found naturally in some plants, but commercial extraction is not feasible. Polyols were first discovered by the isolation of ‘manna’ from the mountain ash tree, and sorbitol was isolated from rowan berries [3,29].
Organic acids: Organic acids are found throughout nature. Citric, lactic, malic and gluconic acids have become largescale food and industrial ingredients. Originally produced from fermentation of sucrose or sugar by-products, they are now mainly produced from fermentation of dextrose.
Amino acids: During the 1980s, advances in fermentation technology allowed the economic production of a number of amino acids from starch hydrolyzes. Examples are lysine, threonine, tryptophan, methionine and cysteine. Starchderived amino acids are generally used as animal nutrition supplements; enabling animal nutritionists to formulate these amino acids via Saccharomyces yeast as substrates. Modern ethanol plants use simultaneous scarification, yeast propagation and fermentation. The major portion of fuel-grade ethanol is now produced by continuous fermentation [3].
Instant starches: Drum-dried, cross linked and stabilized starches became available long time ago, making instant dessert mixes and in-plant cold processing of starchcontaining foods possible. Granular, cold-water-swelling starches appeared in the 1980s, affording much higher quality in instant desserts and cold processing of dressings and other foods. Cold-water-swelling starches were prepared by spray drying and hot aqueous ethanol treatment [16].
Starch has a range of roles in a variety of foods, as shown in Table 2. An understanding of the mechanism underlying each effect is necessary to make the best use of starch in these functions. To gain that understanding, it is helpful to track the changes that starch undergoes during pasting and cooling, and the impact these have on the structures of foods.
| Function | Food Applications |
|---|---|
| Adhesion | Battered and breaded foods |
| Binding | Formed meat, snack seasonings |
| Clouding | Beverages |
| Crisping | Fried and baked foods, snacks |
| Dusting | Chewing gum, bakery products |
| Emulsion | stabilization Beverages, creamers |
| Encapsulation | Flavors, beverage clouds |
| Expansion | Snacks, cereals |
| Fat replacement | Ice cream, salad dressings, spreads |
| Foam stabilization | Marshmallows |
| Gelling | Gum drops, jelly gum centers |
| Glazing | Bakery, snacks |
| Moisture retention | Cakes, meats |
| Thickening | Gravies, pie fillings, soups |
Table 2. Functions and applications of starch in food.
Starch structures relevant to foods
Prior to heating in water, starch granules are insoluble and will absorb only a limited amount of water. Starch paste changes during storage and the resulting effect is on texture and appearance of the foods. The selection of starch for a given use depends on the desired food properties, as well as the processing and distribution stresses involved [3].
During processing, starch granules swell and are fragmented and solubilized to varying degrees, according to the severity of heating and mechanical shear [30]. The distribution of starch between swollen granules, fragmented granules and solubilized polymers determines the texture, appearance and stability of the paste, and the food [31].
Gelatinization and Pasting properties
Both gelatinization and pasting are used to describe changes that starch undergoes when heated in water; clarification of these is helpful for a meaningful discussion. According to, gelatinization is the collapse (disruption) of molecular order within the starch granule manifested in irreversible changes in properties [1]. Such as granular swelling, crystallite melting, loss of birefringence and starch solubilization. The point of initial gelatinization and the range over which it occurs is governed by starch concentration, method of observation, granule type and heterogeneities within the granule population under observation [32].
Gelatinization on a macroscopic scale causes thickening and loss of opacity, which creates a semi-solid from a liquid or solid and may be accompanied by increased turbidity. Pasting, according to is the phenomenon following gelatinization in the dissolution of starch [33-36]. It involves granular swelling, exudation of molecular components from the granule and, eventually, total disruption of granules. By this definition, pasting goes beyond optimal cooking for thickening by starch, but describes what is needed when starch is in the form of dissolved polymer molecules as required for emulsification, gelation and mouth feel. Both gelatinization and pasting are general terms which do not precisely define the condition of processed starch, but include a number of phenomena which occur during starch processing.
Changes during cooking
When heated in water, the starch granule begins to swell as thermal energy breaks hydrogen bonds between adjoining starch polymers. Amorphous regions are disrupted first. Bonds in the crystalline areas hold the granule intact until a point is reached where they are also broken. With continued heating, the granule swells to many times its original volume. Intact swollen starch granules have a major impact on the rheological properties of starch slurry. The physical force or friction between these highly swollen granules and interactions with large solubilized polymers causes the paste to thicken. During granular swelling amylose, if present, leaches from the granule and, if allowed to reassociate in the surrounding solution, makes the paste cloudy or opaque. Amylose can initiate gelation (setback or retrogradation). The gel may shrink and express water and become rubbery. The swollen granule is susceptible to disruption via additional heating or shear. According to granules swell excessively and rupture, a dispersion of amylose, amylopectin and granule fragments is formed and the paste loses viscosity and becomes long [37-40].
Conclusion
There is substantial progress made on various aspects of starches from diverse species. Diversity in composition, structure, modification, applications and other properties of starches within the same species and among diverse species abounds but little generalized features for such a great diversity, especially in modification and application in food and non -food systems. Starch isolation should target yield specifics as some of them can be rich in non-starchy polysaccharides and proteins. There are numerous opportunities to further explore and better utilize starches especially from structure, modification, characterization, Standardization for scaling up extraction and applications. Starch structure–application in relations to food systems such as bread and pasta making and film formation remains a better establishment.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Wurzburg OB. Modified starches: properties and uses. 1986.
- James MG, Denyer K, Myers AM. Starch synthesis in the cereal endosperm. Curr Opin Plant Biol. 2003;6(3):215-22.
- Belitz HD, Grosch W, Schieberle P. Protein chemistry (4th edition). Springer.2009
- Dhull SB, Malik T, Kaur R, et al. Banana Starch: Properties Illustration and Food Applications-A Review. Starch‐Starke. 2021;73(1-2):2000085.
- Kaur L, Dhull SB, Kumar P, et al. Banana starch: Properties, description, and modified variations-A review. Int J Biol Macromol. 2020;165:2096-102
- Singh J, Kaur L, McCarthy OJ. Factors influencing the physico-chemical, morphological, thermal and rheological properties of some chemically modified starches for food applications-A review. Food Hydrocoll. 2007;21(1):1-22.
- Bednar GE, Patil AR, Murray SM, et al. Starch and fiber fractions in selected food and feed ingredients affect their small intestinal digestibility and fermentability and their large bowel fermentability in vitro in a canine mode. J Nutr. 2001;131(2):276-86.
- Englyst HN, Kingman SM, Hudson GJ. Measurement of resistant starch in vitro and in vivo. Br J Nutr. 1996;75(5):749-55.
- Silanere LM, Malleshi NG, Mahadevamma L. Resistant starch from differently processed rice and ragi (finger millet). Eur Food Res Technol.1999;209:32-7.
- Choi SG, Kerr WL. Effects of chemical modification of wheat starch on molecular mobility as studied by pulsed 1H NMR. LWT-Food Sci Technol. 2003;36(1):105-12.
- Kaure L, Singh N, Singh J. Factors influencing the properties of hydroxypropylated potato starches. Carbohydr polym. 2004;55(2):211-23.
- Adebowale KO, Lawal OS. Functional properties and retrogradation behaviour of native and chemically modified starch of mucuna bean (Mucuna pruriens). J Sci Food Agric. 2003;83:1541-46.
- Cornell H. The functionality of wheat starch. Starch in food. 2004:211-40.
- Chatakanonda P, Varavinit S, Chinachoti P. Effectof crosslinking on thermal and microscopic transitions of rice starch. LWT-Food Sci Technol. 2000a;33(4):276-84.
- Chatakanonda P, Varavinit S, Chinachoti P. Relationship of gelatinization and recrystallization of cross‐ linked rice to glass transition temperature. Cereal Chem. 2000b;77(3):315-19.
- Maningat CC, Bassi S. Starch Technology. In: Proceedings of the International Starch Technology Conference. 1999.
- Lawal OS, Lechner MD, Kulicke WM. Single and multi-step carboxymethylation of water yam (Dioscorea alata) starch: Synthesis and characterization. Int J Biol Macromol. 2008;42(5):429-35.
- Wang S, Yu J, Zhu Q, et al. Granular structure and allomorph position in C-type Chinese yam starch granule revealed by SEM, 13C CP/MAS NMR and XRD. Food Hydrocoll. 2009;23(2):426-33.
- Lineback DR, Rasper VF. Wheat carbohydrates. Wheat: chemistry and technology.1999;1(3):277-372.
- Odeku OA, Picker-Freyer KM. Evaluation of the material and tablet formation properties of modified forms of Dioscorea starches. Drug Dev Ind Pharm. 2009;35(11):1389-1406.
- Jiang Q, Gao W, Li X. Comparative susceptibilities to alkali- treatment of A-, B-and C-type starches of Dioscorea zingiberensis, Dioscorea persimilis and Dioscorea opposita. Food Hydrocoll. 2014;39:286-94.
- Alves RML, Grossmann MVE, Silva RSSF. Gelling properties of extruded yam (Dioscorea alata) starch. Food Chem. 1999;67(2):123-7.
- Hoover R, Hughes T, Chung HJ. Composition, molecular structure, properties, and modification of pulse starches: A review. Food Res Int. 2010;43(2):399- 413.
- Takaoka M, Watanabe S, Sassa H. Structural characterization of high molecular weight starch granule- bound proteins in wheat (Triticum aestivum L). J Agr Food Chem,1997;45(8):2929-34.
- Waduge RN, Kalinga DN, Bertoft E. Molecular structure and organization of starch granules from developing wheat endosperm. Cereal Chem. 2014;91(6):578-86.
- Nuwamanya E, Baguma Y, Kawuki RS. Quantification of starch physicochemical characteristics in a cassava segregating population. Afr Crop Sci J. 2008;16(3):191-202.
- Nuwamanya E, Baguma Y, Wembabazi E, et al. A comparative study of the physicochemical properties of starches from root, tuber and cereal crops. Afr J Biotech. 20111;10(56):12018-30.
- Wattanachant S, Muhammad KMAT, Hashim DM. Effect of crosslinking reagents and hydroxypropylation levels on dual-modified sago starch properties. Food Chem. 2003;80(4):463-71.
- Maningat CC, DeMeritt GK, Chinnaswamy R, Bassi SD. Properties and applications of texturized wheat gluten. Cereal Food world. 1999;44(9):650-55.
- Maningat CC, Seib PA. Wheat Quality In: Proceedings of the International Wheat Quality Conference, Manhattan. 1997;261-84.
- Bassi S, Maningat CC, Chinnaswamy R, et al. Washington, DC: U.S. Patent and Trademark Office. 1997.
- Lawal OS, Ogundiran OO, Adesogan EK, et al. Effect of hydroxypropylation on the properties of white yam (Dioscorea rotundata) starch. Starch‐Starke. 2008;60(7):340-8.
- Mahadevamma S, Tharanathan RN. Processing of legumes: resistant starch and dietary fiber contents. J Food Qual. 2009;27(4):289-303.
- Seo CC, Thevamalar K. Internal plasticization of granular rice starch by hydroxypropylation: Effects on phase transitions associated with gelatinization. Starch‐ Starke. 1993;45(3):85-8.
- Singla D, Singh A, Dhull SB. Taro starch: Isolation, morphology, modification and novel applications concern-A review. Int J Bio Macromol. 2020;163:1283-90.
- Son TK, Joo LC, Jun CS. Hydrothermal Treatment of Water Yam Starch in a Non‐ granular State: Slowly Digestible Starch Content and Structural Characteristics. J Food Sci. 2012;77(6):C574-C582.
- Tattiyakul J, Naksriarporn T, Pradipasena P. Effect of moisture on hydrothermal modification of yam Dioscorea hispida Dennst starch. Starch‐Stärke. 2006;58(3‐4):170-6.
- Tester RF, Morrison WR. Swelling and gelatinization of cereal starches. II. Waxy rice starches. Cereal Chem. 1990;67(6):558-63.
- Wang MQ, Xu ZR, Sun JY. Effects of enzyme supplementation on growth, intestinal content viscosity, and digestive enzyme activities in growing pigs fed rough rice-based diet. Asian-Aus J Ani Sci. 2008;21(2):270-6.
- Whistler RL, BeMiller JN, Paschall EF, editors. Starch: chemistry and technology. Academic Press; 2012.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref