Research Article - Biomedical Research (2017) Volume 28, Issue 2
Stachydrine ameliorates the cerebral ischemia by inhibiting the activity of histone deacetylase in neonatal rats
Zhang L1, Zheng N2, Ma C1, Duan R3*1Department of Intensive Care Unit, the Second Hospital of Shandong University, China
2Department of Clinical Laboratory, Shandong Provincial Hospital Affiliated to Shandong University, China
3Department of Neurology, Shandong Provincial Qianfoshan Hospital, Shandong University, China
- *Corresponding Author:
- Duan R
Department of Neurology
Shandong Provincial Qianfoshan Hospital
Shandong University, China
Accepted date: July 26, 2016
Abstract
Cerebral ischemia is the commonest cause of death throughout the world. Present investigation deals with the effect of stychedrine on cerebral ischemia induced injury by inhibiting the activity of Histone Deacetylase (HDAC) in neonatal rats. Cerebral ischemia was produced by ligation of carotid artery in neonatal rats. After ligation stachydrine (5 and 10 mg/kg) was given by IP injection in neonatal rats for the period of five days. Infract volume, Apoptosis, HDAC activity, expression of acetylated H3 and H4 proteins, markers of oxidative stress [Superoxide Dismutase (SOD) and Glutathione Peroxidase (GSHPx)] and cytokines were estimated in the cerebral tissues of cerebral ischemic rats. The result suggested that stachydrine treatment significantly (p<0.01) reduced the infract volume, apoptosis ratio, HDAC activity, markers of oxidative stress and cytokines level in the brain tissues of cerebral ischemic neonatal rats compared to negative control group. Moreover it improves the expression of H3 and H4 in the brain tissue of cerebral ischemic neonatal rats. The given study concludes that stachydrine ameliorates the cerebral ischemia in neonatal rats by inhibiting HDAC activity.
Keywords
Stychedrine, Histone deacetylase (HDAC), Oxidative stress, Cerebral ischemia.
Introduction
Hypoxia is the commonest cause of physical disability and death due to brain damage in neonates during before and after birth [1]. Brain is a visceral organ and decreased in the blood flow results in neuronal damage, inflammation and cerebral ischemia. Literature suggested that 60% of premature babies are suffering from cerebral ischemia associated with mental abnormalities [2]. Hypoxia produces stress on the foetus during the early developmental stage and thereby increases in oxidative stress and inflammation of neurons which leads to neuronal damage [3,4]. Histone Deacetylase (HDAC) is the enzymes which control the expression of gene and functions of different proteins. HDAC inhibitor decreases the expressions of inflammatory mediators like p53, cytokinins and NFκB [5]. Moreover previous study reported the neuroprotective effect of HDAC inhibitors and justifies the role of HDAC inhibitors as a neuroprotactant on the brain of neonatal rats. Literature also reveals that HDAC inhibitor possesses neuroprotective effect as it improves the number of axons and increases of olegodendrocytes [6]. In the recent year so many naturally derived products were investigated for their neuroprotective activity against the cerebral ischemia induced injury [7,8]. Stachydrine is an alkaloid isolated from Leonurus cardiaca L. (Lamiaceae). Previous studies reported that stachydrine possesses a strong anticancer activity, hypoglycemic and in cardiovascular diseases [9,10]. Reports also suggested protective effect of stachydrine on endothelial and it also ceases the synthesis of inflammatory mediators [11]. A fluorimetric assay identified the HDAC inhibitors from an herbal extract Leonurus cardiaca L. by enzymatic assay [12]. On the basis of this the present study investigates the neuroprotective effect of stachydrine by inhibiting HDAC in neonatal rats.
Materials and Methods
Animal
Sprague-Dawley rats pregnant were procured from the Laboratory Animal Department of China Medical University and laboratory was kept under controlled condition as per the guidelines (cycle of 12 h light/day, pathogen free). All the protocol of the given study was approved by institutional ethical committee (V. No: 109/2014). Stachydrine was procured from sigma Aldrich (USA).
Induction of brain injury
Pups (P7) were properly anesthetized by injection of Phenobarbital and permanent ligation was done by exposing the carotid artery. Thereafter all the neonatal rats were placed in the dam and recovered pups was exposed to a hypoxia condition by placing it in an incubator (37°C, 8% O2 and 92% nitrogen) for the period of one hour. The highest tolerance period of hypoxia was estimated by the preliminary study. Temperature of the chamber was kept constant at 37°C and moderately submerged into water. Pups were kept 5 min for recovery after one hour of hypoxia. All the pups were divided in to four groups as follows: Control (normal rats), Negative control (Saline treated cerebral ischemic rats, n=6), stachydrine treated cerebral ischemic rats (5 and 10 mg/kg, ip, n=6). As per the protocol all the groups were given treatment for the period of five days [13]. At 12 h later the treatment protocol (P12) body weight and temperature were estimated and brain of all the anesthetized (phenobarbitone) pups was isolated by intracardial perfusion with saline. The isolated brain separated in to two hemicerebrum and one of them was stored into frozen liquid nitrogen at -70°C for the estimation of protein. Other side of the isolated hemicerebrum was applied to TUNEL staining [14].
Estimation of infract volume
Infracted volume was estimated by using 2, 3, 5-Triphenyl Tetrazolium Chloride (TTC) in saline solution for the period of 15 min. Analysis of Images of brain sections was achieved by using computerized image analyzer. Infracted area of slices of the brain of same hemisphere added together to get total infract volume [15].
Evaluation of apoptosis
Brain section were embedded in paraffin and the section was deparaffinized by dipping it into xylene (5 min) and 70, 95, 100% ethanol for hydration. Then tissue was incubated for 5 min with proteinase k (20 g/ml), situ apoptosis estimation kit (Roche Applied Science, Germany) used for the TUNEL method. The tissue sections were cleaned with buffer and thereafter incubated for 30 min. Tissue sections were again stained by mounting it into hematoxylin and then rinse it with water. After dehydration in graded ethanol series and transparent in xylene, the brain sections were mounted onto gelatin-coated slides. The ratio of apoptotic neurons to the total number of neurons was considered as % of apoptotic neurons. The estimation of apoptotic neurons were achieved by histopathological analysis [16].
Tissue preparation for protein analysis
Frozen hemicerebrum of all the pups were homgenated with ice cold buffer. The composition of buffer is 2 mM EDTA and protease inhibitor (1%) mixed in Tris Buffer (TBS). the fraction of nuclear protein was extracted out by centrifuging it at 800 x for the period of 10 min and cytosolic protein by centrifuging at 9200 x for the period of 15 min. the supernatant was used for the estimation of protein.
Estimation of HDAC activity
Colorimetric detection was used for the estimation of HDAC activity by using kit (Upstate, CA). The absorbance at 405 nm was observed and the HDAC activity was expressed in terms of the % of control group. Effect of stachydrine on expression of acetylated H3 and H4 was estimated by using western blot analysis [17].
Estimation of biochemical analysis
The supernatant of tissue homogenate was used for the estimation of cytokinins by specific Enzyme-Linked Immunosorbent Assay (ELISA) kits [18] and the activity of Superoxide Dismutase (SOD) and Glutathione Peroxidase (GSH-Px) in the brain tissues of all the pups estimated by spectrophotometer (Shimadzu, Japan) [17].
Statistical analysis
Results
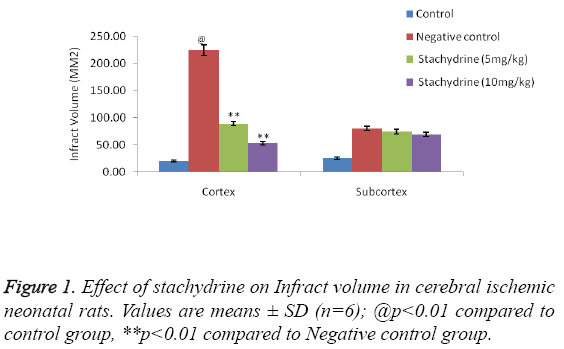
Effect of stachydrine on infract volume
The effect of stachydrine on the infract volume of cerebral ischemia induced injury in neonatal rats shown in Figure 1. It was observed that hypoxia condition results in increased infract volume in negative control group of rats compared to control group (shame operated). Treatment with stachydrine (5 and 10 mg/kg) significantly (p<0.01) decreases the infract volume compared to negative control group of rats. However the infract volume at subcortex region remain unchanged in all the animals of cerebral ischemia induced injury in neonatal rat model.
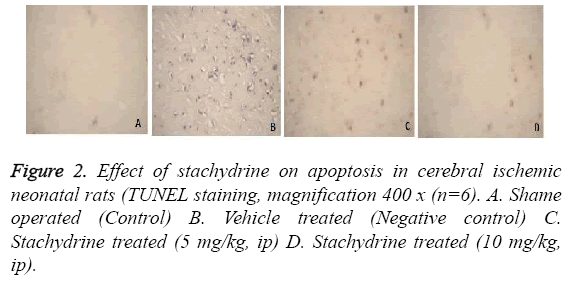
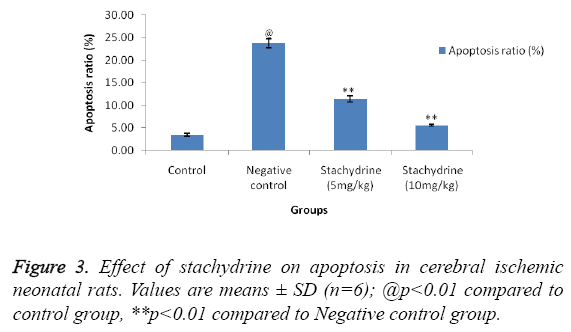
Effect of stachydrine on apoptosis in cerebral ischemic neonatal rats
Effect of stachidrine on apoptosis in the brain tissues of cerebral ischemic neonatal rats was shown in Figures 2 and 3. For the estimation of apoptosis TUNEL staining was done on cerebral tissues of neonatal rats as shown in Figure 2. TUNEL staining reported to show clear, visible staining of nuclei and its fragments in hypoxia induced cerebral injury in neonatal rats. Whereas, staining were found to be faint or less visible in the group treated with stachydrine compared to negative control group. This reduction in apoptosis ratio by stachydrine ameliorates the injury in cerebral tissues of hypoxic neonatal rats.
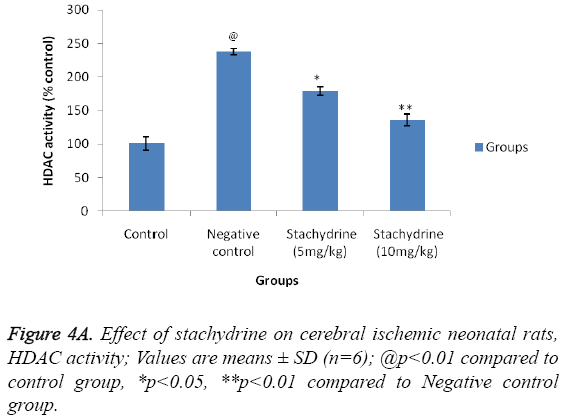
Effect of stachydrine on HDAC activity
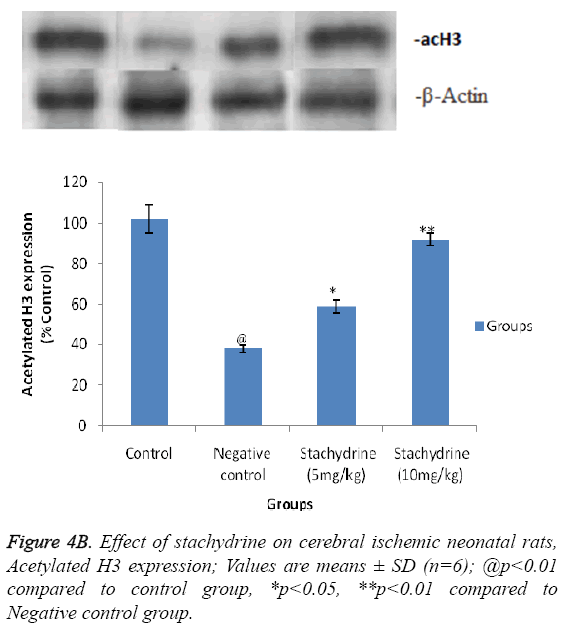
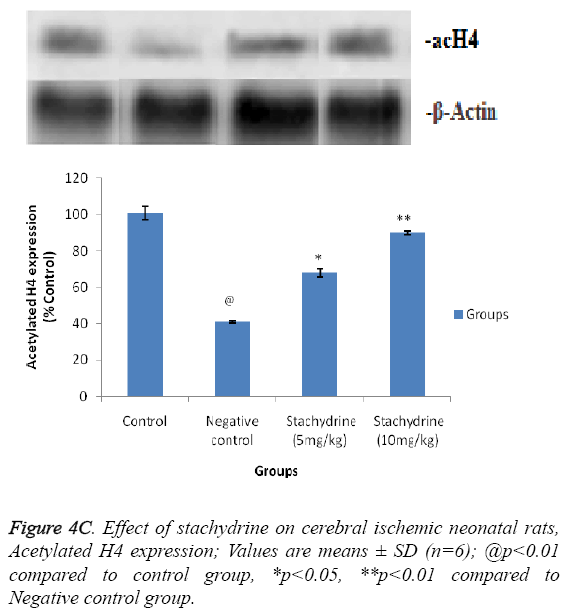
Hypothesis of our study was confirmed by estimating the effect of stachydrine on HDAC activity in the brain tissues of ischemia induced injury in neonatal rats as shown in Figure 4A. It was observed that stachydrine decreases the activity of HDAC enzyme in dose dependent manner in the brain tissues of ischemia induced injury in neonatal rats. Moreover the effect of stachydrine on the expression of acetylated H3 and H4 protein in the brain tissues of ischemic neonatal rats was determines in this study (Figures 4B and 4C). There were significant (p<0.01) increases in the expression of acetylated H3 and H4 proteins with the stachydrine treatment in cerebral tissues of ischemic neonatal rats compared to negative control group.
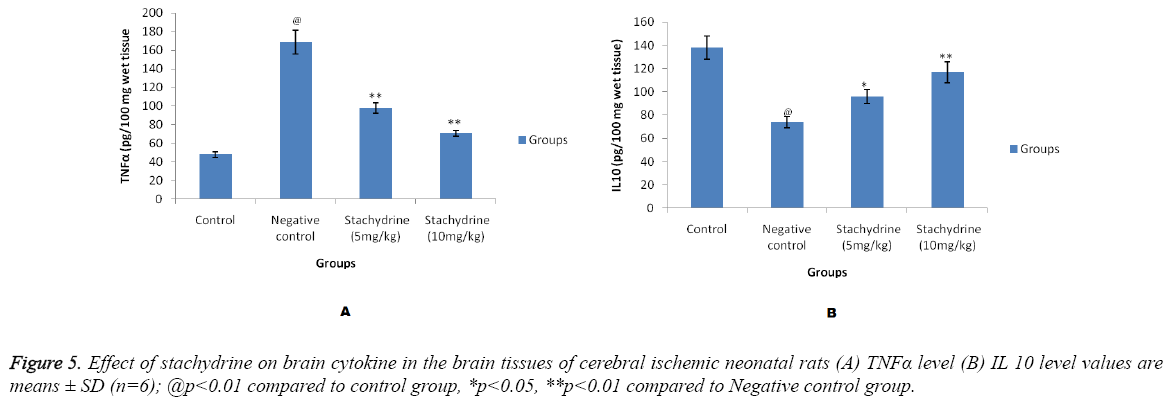
Estimation of cytokines in the brain tissues
Figure 5 shows effect of stachydrine on the cytokine level in brain tissues of cerebral ischemic neonatal rats. There were significant (p<0.01) increase in the level of TNFα in the brain tissues which confirms the brain injury induced by cerebral ischemia in neonatal rats compared to control group. Whereas, stachydrine ameliorates the hopoxia induced cerebral injury by significant (p<0.01) reduction in the level of TNFα compared to negative control group of neonatal rats (Figure 5A). Moreover the level of IL 10 found to be increased significantly (p<0.05, p<0.01) in stachydrine treated group of negative control group (Figure 5B). Effect of stachydrine on the TNFα and IL 10 were found to be in a dose dependent manner.
Estimation of oxidative stress in the brain tissues
Markers of oxidative stress parameters such as SOD and GSH in the brain tissues of hypoxia induced cerebral injured neonatal rats were found to be decreased compared to control group of rats. It was observed that treatment with the stachydrine significantly increases (p<0.01) in the SOD and GSH-PX level in the brain tissues and thereby it decreases the oxidative stress in cerebral ischemic neonatal rats as shown in Table 1.
| Sr. No. | Group | SOD (U/100 mg wet tissue) | GSH-PX (U/100 mg wet tissue) |
|---|---|---|---|
| 1 | Control | 2.67 ± 0.23 | 192.7 ± 8.3 |
| 2 | Negative control | 0.79 ± 0.12@ | 96.8 ± 5.9@ |
| 3 | Stachydrine 5 mg/kg, ip | 1.73 ± 0.19** | 130.6 ± 7.2** |
| 4 | Stachydrine 10 mg/kg, ip | 2.18 ± 0.20** | 169.2 ± 8.1** |
Table 1: Effect of Stachydrine on parameters of oxidative stress in the brain tissues of cerebral ischemic neonatal rats.
Discussion
Present investigation deals with the neuroprotective effect of stachydrine in cerebral ischemic neonatal rats by inhibiting the HDAC activity in the cerebral tissues. Acetylation of histone is found to play role in different cognitive function. It was also observed that during learning the acetylation of histone increase in the brain tissues of rodent [6]. Various studies were performed for the protective possibilities in the management of cerebral ischemia [19]. In this study, we had produced the cerebral ischemia in neonatal rats. Literature suggested that HDAC inhibitors possess the neuroprotectant property by its action of reducing neuro inflammation and oxidative stress in neonatal rats [20]. Hypoxia in neonatal rats develops infract in the brain tissue as there was increase in the neuronal apoptosis. It was observed that stachydrine treatment significantly decreases (p<0.01) the infract volume and apoptosis ratio in cerebral ischemic neonatal rat, which confirms its Neuroprotective effect. Previous studies reported that cerebral ischemia alters the mitochondrial function of cell and lead to increase in the production of reactive oxygen species and generates the free radical. It also alters the level of cytokines in the brain tissue. Several herbals having antioxidant properties which posse’s therapeutic effect [21]. These alterations reported to damage the brain cell in cerebral ischemia [22]. However, stachydrine treatment decreases (p<0.01) oxidative stress and alters the level of brain cytokine. It also decreases the HDAC activity and improves the expression of H3 and H4 proteins in brain tissues of cerebral ischemic neonatal rats. Decrease in the HDAC activity and increase in the expression of H3 and H4 proteins in brain tissues by stachydrine treatment confirm the possible mechanism of its Neuroprotective effect [14,15]. On the basis of all of the above property stachydrine reverse the cerebral damage induced by hypoxia in neonatal rats.
Conclusion
The present investigation concludes the neuroprotective effect of stachydrine in cerebral ischemia in neonatal rat. It also postulates the mechanism of its action by inhibiting HDAC activity and thus decreases the oxidative stress and neuroinflamation in cerebral ischemia in neonatal rats.
References
- Moskowitz MA, Lo EH, Ladecola C. The science of stroke: mechanisms in search of treatments. Neuron 2010;67:181-198.
- Kurinczuk J, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic–ischaemic encephalopathy. Early HumDev2010;86: 329-338.
- Rodriguez PJ, Xiong F, Li Y, Zhou J, Zhang L. Fetal hypoxia increases vulnerability of hypoxic–ischemic brain injury in neonatal rats: Role of glucocorticoid receptors.Neurobiol Dis 2014; 65: 172-179.
- Chen PS, Peng GS, Li G, Yang S, Wu X, Wang CC, Wilson B, Lu RB, Gean PW,Chuang DM, Hong JS. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. MolPsychiatry 2006; 11:1116-1125.
- Halili MA, Andrews MR, Sweet MJ, Fairlie DP. Histone deacetylase inhibitors ininflammatory disease. Curr Top Med Chem 2009; 9: 309-319.
- Fleiss B, Nilsson MK, Blomgren K, Mallard C.Neuroprotection by the histonedeacetylase inhibitor trichostatin A in a model of lipopolysaccharide-sensitised neonatal hypoxic-ischaemic brain injury. J Neuroinflammation2012; 9:70.
- Ahmed MA, ElMorsy EM, Ahmed AA. Pomegranate extract protects against cerebral ischemia/reperfusion injury and preserves brain DNA integrity in rats. Life Sci2014; 110: 61-69.
- Aguilera P, Chanez-Cardenas ME, Ortiz-Plata A, Leon-Aparicio D, Barrera D, Espinoza-Rojo M, Villeda-Hernandez J, Sanchez-Garcia A, Maldonado PD. Aged garlic extract delays the appearance of infarct area in a cerebral ischemia model, an effect likely conditioned by the cellular antioxidant systems.Phytomedicine 2010;17:241-247.
- Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J ClinNutr 2003; 78:559S-569S.
- Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch InternMed 2009; 169:659-669.
- Hu YY, He KW, Guo RL. Six alkaloids inhibit secretion of IL‐1a, TXB(2), ET‐1, andE‐selectin in LPS‐induced endothelial cells. Immunol Invest 2012; 41:261-274.
- Krasteva S, Heiss E, Krenn L. Optimization and application of a fluorimetric assay for the identification of histone deacetylase inhibitors from plant origin. Pharm Biol2011; 49:658-668.
- Roohey T, Raju TN.Moustogiannis AN: Animal models for the study of perinatalhypoxicischemic encephalopathy: A critical analysis. Early Hum Dev 1997;47:115-146.
- Kabakus N, Ay I, Aysun S, Soylemezoglu F, Ozcan A, Celasun B. Protective Effects of Valproic Acid Against Hypoxic-Ischemic Brain Injury in Neonatal Rats. J Child Neurol2005; 20: 582-587.
- Turkan K, Mesut M, Bulent G, Merih C, Mehmet C, Tulin A. Uridine protects against hypoxic-ischemic brain injury by reducing histone deacetylase activity in neonatal rats. RestorNeurolNeurosci2015; 33: 777-784.
- Zhang Q, Qian Z, Pan L, Li H, Zhu H. The traditional Chinese medicine Huang-Lian-Jie-Du-Tang inhibits hypoxia-induced neuronal apoptosis. Afr J PharmPharmacol 2011; 5: 2558-2565.
- Cetinkaya M, Cansev M, Kafa IM, Tayman C, Cekmez F, Canpolat FE, Tunc T, Sarici SU. Cytidine 5'-diphosphocholine (CDP-choline) ameliorates hyperoxic lung injury in a neonatal rat model. Pediatr Res 2013; 74: 26-33.
- Ozdulger A, Cinel I, Koksel O, Cinel L, Avlan D, Unlu A, Okcu H, Dikmengil M, Oral U. The protective effect of N-acetylcysteine on apoptotic lung injury in cecal ligation and puncture-induced sepsis model. Shock 2003; 19: 366-372.
- Palmer C, Roberts RL, Bero C. Deferoxamineposttreatment reduces ischemic brain injury in neonatal rats. Stroke 1994;25:1039-1045.
- Yildirim F, Gertz K, Kronenberg G, Harms C, Fink KB, Meisel A, Endres M. Inhibition of histone deacetylation protects wildtype but not gelsolindeficient mice from ischemic brain injury. ExpNeurol 2008; 210:531-542.
- Lalrinzuali K, Vabeiryureilai M, Jagetia GC, Lalawmpuii PC. Freeradical scavenging and antioxidant potential of different extracts of Oroxylumindicum in vitro. Adv Biomed Pharma2015; 2: 120-130.
- Ahmed MA, ElMorsy EM, Ahmed AA. Pomegranate extract protects against cerebral ischemia/reperfusion injury and preserves brain DNA integrity in rats. Life Sciences 2014; 110: 61-69.