Research Article - Current Pediatric Research (2023) Volume 27, Issue 12
Spinal anesthesia in pediatric surgical patients with high risk of perioperative respiratory adverse events: Experience at a tertiary care hospital of north India with limited resource settings.
Shafat A Mir1* , Majid Jehangir1 , Raashid Hamid2 , Rashid Javad Fazli1, Akshat Sudhanshu Mushtaq1 , Altaf Hussain Mir1, Khalid Sofi2*, Sahu Mushtaq1 , Altaf Hussain Mir1, Khalid Sofi1
1Department of Anesthesiology, Sheri Kashmir Institute of Medical Science, Srinagar, Jammu and Kashmir, India
2Department of Paediatric and Neonatal Surgery, Sheri Kashmir Institute of Medical Science, Srinagar, Jammu and Kashmir, India
- Corresponding Author:
- Shafat A Mir,
Department of Anesthesiology, Sher-I-Kashmir Institute of Medical Sciences, Srinagar, Jammu and Kashmir, India
E-mail: mir.shafatahmad@gmail.com
- Akshat Sudhanshu,
Department of Paediatric and Neonatal Surgery, Sheri Kashmir Institute of Medical Science, Srinagar, Jammu and Kashmir, India
E-mail: akshat.sudhanshu108@gmail.com
Received: 27 November, 2023, Manuscript No. AAJCP-23-123427; Editor assigned: 29 November, 2023, Pre QC No. AAJCP-23-123427 (PQ); Reviewed: 14 December, 2023, QC No. AAJCP-23-123427; Revised: 21 December, 2023, Manuscript No. AAJCP-23-123427 (R); Published: 29 December, 2023, DOI:10.35841/0971-9032.27.10.1185-1189.
Abstract
Background: In the pediatric population, Spinal Anesthesia (SA) has been used sporadically since it is usually saved for patients whose perceived risk of general anesthesia is high because of coexisting disorders.
Objectives: The objective of this study was to evaluate the feasibility,safety and hemodynamic stability related to pediatric spinal anesthesia.
Methods: In this 5-year prospective study, 112 paediatric patients aged 1 month to 5 years of age were recruited. Spinal anesthesia was administered using hyperbaric bupivacaine 0.5% in a dose of 0.5 mg/kg (for child <5 kg), 0.4 mg/kg (for 5-15 kg), 0.3 mg/kg (for >15 kg) in L4–L5 space under all aseptic precautions after sedation. Demographic data, risk factor assessment, vital parameters, supplemental sedation, sensory-motor block characteristics, and complications if any, were noted.
Results: A total of 112 spinal anesthesia were performed in infants and children up to 5 years of age. The mean duration of the surgery was 45.21 ± 14.86 minutes. Hemodynamic parameters (SBP, DBP, HR, and Spo2) remained stable at all points of time during perioperative period. Inhaled sevoflurane was the most common (n=80, 83%) sedative used for the procedure. No perioperative complications related to SA were observed.
Conclusion: Spinal anesthesia is a safe and effective alternative to general anesthesia in pediatric patients who are high risk for GA.
Keywords
Spinal anesthesia, Adverse events, Pediatric patients.
Introduction
Roughly 15% of children under General Anesthesia (GA) have perioperative respiratory adverse effects; rates as high as 50% have been documented for certain common surgical procedures [1]. Increased airway reactivity is linked to a higher frequency of perioperative respiratory adverse events, and this relationship is most pronounced in children who have asthma, eczema, a recent upper respiratory tract infection, or passive smoke exposure [2]. Adverse respiratory events during surgery are linked to a higher likelihood of extended hospital stays, which negatively affects patients and their families, lengthens surgery wait times, and raises healthcare expenses [3]. There is paucicity of high dependency units and ICU beds in limited resource settings, which often leads to delay in surgical procedures.
Our department created a program wherein Spinal Anesthesia (SA) is utilized instead of general anesthesia for brief surgical operations in response to above mentioned concerns expressed by parents and surgeons. Infraumbilical and lower limb procedures benefit greatly from the use of spinal anesthetic, particularly in infants and young children who are more vulnerable to complications from GA [4,5]. We report the use of SA instead of general anesthesia in a cohort of 112 infants and children undergoing various brief surgical procedures, describing our protocols and dosing regimens, success rate, hemodynamic stability, and discuss the potential adverse effects related to this technique.
Materials and Methods
After approval of Intra Departmental Ethical Committee this study was conducted in the Department of Anesthesiology, Sher-I-Kashmir Institute of Medical Sciences, Srinagar (SKIMS), and India from June 2018 to June 2023. SKIMS are a tertiary care referral centre for the management of high risk pediatric patients.
Inclusion criteria
Pediatric patients up to 5 years of age with at least two risk factors listed in Table 1 as reported by parents/guardians and who were scheduled for elective surgery were enrolled for the study.
| Respiratory | Non respiratory |
|---|---|
| Respiratory tract infection | Eczema |
| Asthma/hay fever | Passive smoking |
| Congenital airway anomalies | Prematurity |
| Congenital heart disease |
Table 1. Risk factors for respiratory adverse events.
Exclusion criteria
• Any contraindication to lumbar puncture.
• Parent/guardian refusal.
Informed consent was obtained from parents of each patient for participation in the study. This resulted in sample size of 112 patients. Pre anesthesia evaluation was done a day before the surgery as per the standard departmental policy. All patients were kept fasting for 6 hrs to solid foods and 2 hrs for clear fluids prior to Anesthesia. EMLA cream was used 45 min before arrival to operating room on lumbar puncture and venipuncture sites. On arrival to operating room standard anesthesia monitoring was instituted i.e. electrocardiography, non-invasive blood pressure measurements, capnography, and pulse oximetry.
Hemodynamic parameters including heart rate, systolic and diastolic blood pressure, and oxygen saturation were measured and noted as baseline values. Ringer lactate 10 ml/kg was given to all patients before the procedure. All patients were premedicated with injection glycopyrollate 10 μg/kg. In order to provide an immobile patient for spinal block, patients were sedated with ketamine 1 mg/kg, fentanyl 1-2 mcg/kg, midazolam 0.03 mg/kg IV or inhaled sevoflurane (8%). Midline approach was used in all patients in lateral or sitting position under aseptic precautions at L4-L5 interspace using standard 25G or 27G pediatric quincke spinal needles.
We used hyperbaric bupivacaine 0.5% in a dose of 0.5 mg/kg (for child <5 kg), 0.4 mg/kg (for 5-15 kg), 0.3 mg/kg (for >15 kg). The dosage used was based on various previous studies of spinal anesthesia in children. All the procedures were done by trained consultant anesthesiologists. Sensory level was assessed by lack of response to firm skin pinch [6]. Attainment of T10 dermatomal sensory level was considered as successful block. Motor block was assessed with Bromage score [7]. Bromage 3(complete): Unable to move feet or knees, Bromage 2(almost complete): Able to move feet only, Bromage 1(partial): Just able to move knees, Bromage 0(None): Full flexion of knees and feet. Peak sensory level of at least T10 and Bromage score of 3, after 10 minutes of block was considered as successful block. Otherwise, case was designated as failure and was converted to GA. After the surgery, patients were shifted to Post Anesthesia Care Unit (PACU) for monitoring of vitals and regression of block. Adverse events if any were recorded.
Measurements
Demographic data including age and sex. Type and duration of surgery were noted. Hemodynamic parameters viz heart rate, systolic and diastolic blood pressure and oxygen saturation were recorded at baseline and then at 5, 15, 30, 45, 60 minutes after the block. Sensory and motor block characteristics, spinal needle; lumbar puncture related characteristics, failure rate, block regression and complication, if any were also recorded.
Presentation of data
The continuous variables are expressed in mean ± standard deviation and the categorical variables in frequencies.
Results
Over a period of 5 years a total of 112 spinals anesthesia were indicated in a predominantly male dominated (73.2%) study group. The demographic data of the study cohort along with the type of surgical procedures are listed below (Table 2). The mean duration of the surgery was 45.21 ± 14.86 minutes. The distribution of risk factors for perioperative respiratory adverse effects is depicted in Figure 1, with recent or active upper respiratory tract infection as predominant factor.
| Mean age (yrs) | 3.12 ± 1.40 |
| Mean weight (kg) | 10.54 ± 3.82 |
| Male/female (n%) | 82 (73.2)/30(26.7) |
| Type of surgery (n%) | |
| Herniotomy | 32 (28.5) |
| Orchidectomy | 24 (21.4) |
| Appendicectomy | 10 (8.9) |
| Pylorotomy | 17 (15) |
| Lower limb surgery | 15 (13.3) |
| Urological surgery | 14 (12.5) |
| Mean duration of surgery (min) | 45.21 ± 14.86 |
Note:Data presented as mean± standard deviation and percentage.
Table 2. Demographic data.
There was no significant change in the mean value of systolic blood pressure, diastolic blood pressure, heart rate, respiratory rate, and oxygen saturation after subarachnoid block at all time periods (Table 3). On the operation table, most of the patients (n=96, 85.71%) received sedative drug to prevent any untoward movement during SAB. Inhaled sedation was given in 80(83%), rest were given ketamine (n=10,(10%) and midazolam(n=6, 6.25%). The various characteristics of spinal anesthesia are mentioned in Table 4.
| Variable | 0 min | 5 min | 15 min | 30 min | 45 min | 60 min |
|---|---|---|---|---|---|---|
| SBP | 94.64 ± 7.84 | 92.86 ± 8.34 | 91.22 ± 6.54 | 92.21 ± 5.67 | 91.45 ± 4.36 | 91.35 ± 5.60 |
| DBP | 52.32 ± 4.42 | 50.12 ± 4.78 | 51.46 ± 5.56 | 52.88 ± 5.24 | 52.34 ± 4.77 | 53.45 ± 5.06 |
| HR | 110.2 ± 10.22 | 112.3 ± 9.88 | 106.8 ± 7.55 | 104.6 ± 6.45 | 102 ± 5.78 | 98.45 ± 6.22 |
| RR | 24.24 ± 5.23 | 23.88 ± 4.60 | 22.76 ± 4.12 | 20.54 ± 4.89 | 21.11 ± 4.02 | 20.76 ± 4.89 |
| Spo2 | 97.21 ± 1.24 | 98.23 ± 1.02 | 97.44 ± 0.56 | 97.56 ± 1.10 | 98.42 ± 0.54 | 97.45 ± 1.34 |
Table 3. Hemodynamic parameters under spinal anesthesia.
| Position of patient (n%) | |
|---|---|
| Lateral | 92 (82.1) |
| Sitting | 20 (17.8) |
| No. of punctures | |
| 1 | 78 (69.6) |
| 2 | 25 (22.3 |
| ≥ 3 | 9 (8) |
| Spinal needle used | |
| 25 G | 80 (71.4) |
| 27 G | 32 (28.6) |
| Failure/conversion to GA | 7 (6.25) |
| Sensory block | |
| Mean peak sensory level | T 6.10 ± 1.32 (T4-T8) |
| Time to two segement regression | 46.32 ± 9.6 |
| Motor block | |
| Modified bromage of 3 | 105 (93.75) |
| Time to return to bromage 0 (min) | 90.45 ± 14.67 |
Table 4. Spinal anesthesia characteristics.
There was no need of complementary local anesthesia during the procedures. The SA failure rate was 6.25% and those cases were given general anesthesia. The patients stayed in Post Anesthesia Care Unit (PACU) for 40 (30–70) minutes. We did not observe any perioperative complications in our study cohort.
Discussion
Spinal anesthesia in children is primarily indicated when general anesthesia presents a high risk for respiratory adverse effects like laryngospasm, bronchospasm, cough, post op stridor and apnea [8,9]. Spinal anesthesia is a safe alternative when tracheal intubation presents a significant risk in the perioperative period. Because spinal anesthesia uses fewer perioperative resources, it is particularly appealing in "precarious" or "difficult" conditions. [10]. The latter is especially important in developing countries with high turnover and limited resource settings. Children with respiratory risk factors constitute a large fraction of surgical patients reporting to operating rooms for anesthesia and presenting anesthesiologists with unique challenges especially in developing countries with limited resources.
Hence we focused on this particular subset of children unlike a broader unselected population. In the present study, majority of recruited pediatric patients scheduled for surgery present with symptoms of an Upper Respiratory Tract Infection (URTI). A large number of studies have reported similar numbers for children with a recent (2 to 4 weeks) respiratory tract infections presenting for surgery [11,12]. In Limited resource settings anaesthesiologists may proceed with the surgery as delaying surgery is either not feasible or may not necessarily reduce the risk of perioperative respiratory adverse events [13].
In the absence of adequate number of PICU beds and high dependency units, it is, therefore, critical that the anesthesia management has to be modified in order to minimize the risk of perioperative respiratory adverse events. Airway inflammation and its exacerbation with airway manipulation are considered to be the main reason behind respiratory complications following general anesthesia. Spinal anesthesia is a feasible technique for lower abdominal and lower limbs surgeries under 90 minutes duration [14]. The surgeries in the present study were mostly surgical procedures lasting less than 60 minutes.
It is speculated that the drug uptake is faster in the subarachnoid space in pediatric age owing to proportionally greater blood flow to the spinal cord as compared with adults [15]. This together with faster drug distribution and elimination causes a decreased duration of block in pediatric patients. That is why spinal anesthesia is therefore generally restricted to 1 hr duration surgeries only. Duration, however, can be prolonged with the addition adjuvants like opioids and clonidine [16]. All our patients remained hemodynamically stable after the spinal block till discharge from recovery. This may be due to immature sympathetic nervous system and relatively small intravascular volume in the lower extremities and splanchnic system, in children less than 5 years of age [17].
Most of the patients in our study did not require additional sedation during the procedure. Subarachnoid block itself has been reported to be having the sedative effect. Hermanns et al., [18] conducted a study to evaluate sedation during spinal anesthesia in infants and concluded that SA causes decreased afferent conduction to reticulo-thalamo-cortical projection pathways which reduces the excitability and arousal level of brain. In order to limit the performance bias all the blocks were performed by the same anesthesia team with good experience on this technique. Williams et al., [19] reported a success rate of 97.4%, quite similar with our results. In the present study, no supplementary local anesthesia by the surgeon was required and the GA conversion was 6.25%. A lot of studies in the literature show failure rates between 1.04 to 24.6% which is similar to our study [20,21].
Conclusion
Spinal anaesthesia is a very useful technique for brief surgical procedures in premature and ex-premature patients. However, it may also be used outside this indication and can be a viable technique to include in pediatric surgical patients who are high risk for GA. Our study concluded that SA is a safe and effective alternative to GA in pediatric patients who are high risk for GA related respiratory complications, with better preservation of hemodynamics and free of SA and drug related complications.
Limitations
This was a monocentric study with a selected subgroup of patients. Comparative studies of SA with general anesthesia in such high risk groups needs to be undertaken to establish a definitive correlation with respect to perioperative outcomes.
Source of Funding
Nil
Conflict of Interest
None declared
Institutional Review Board Statement
The intra departmental ethical review board at SKIMS hospital approved our study (SIMS/ANESTH/RP/30 of 2018).
Informed Consent Statement
Informed consent was obtained from parents/guardians of subjects involved in this study.
References
- Murat I, Constant I, Maud'huy H. Perioperative anaesthetic morbidity in children: A database of 24,165 anaesthetics over a 30-month period. PaediatrAnaesth 2004; 14(2):158-66.
- Von Ungern-Sternberg BS, Boda K, Schwab C, et al. Laryngeal mask airway is associated with an increased incidence of adverse respiratory events in children with recent upper respiratory tract infections. Anesthesiology 2007;107(5):714-9.
- Oofuvong M, Geater AF, Chongsuvivatwong V, et al. Excess costs and length of hospital stay attributable to perioperative respiratory events in children. AnesthAnalg 2015;120(2):411-9.
- Somri M, Gaitini LA, Vaida SJ, Malatzkey S, et al. The effectiveness and safety of spinal anaesthesia in the pyloromyotomy procedure. PaediatrAnaesth 2003;13:32–7.
- Shenkman Z, Hoppenstein D, Litmanowitz I, et al. Spinal anesthesia in 62 premature, former-premature or young infants-technical aspects and pitfalls. Can J Anaesth 2002;49(3):262–9.
- Williams RK, Adams DC, Aladjem EV, et al. The safety and efficacy of spinal anesthesia for surgery in infants: The Vermont infant spinal registry. AnesthAnalg 2006;102:67–71.
- KarninaR, Rahayu NS, Faruk M. Factors influencing Bromage score in post-spinal anesthesia patients. Bali Medical Journal 2022; 11(3): 1146-1150. [Crossref][Google Scholar][Indexed]
- Lopez T, Sanchez FJ, Garzon JC, et al. Spinal anesthesia in pediatric patients. Minerva Anestesiol 2012;78(1):78–87.
- Von Ungern-Sternberg BS, Boda K, Chambers NA, et al. Risk assessment for respiratory complications in paediatric anaesthesia: A prospective cohort study. Lancet 2010; 376(9743):773-83.
- Komorowski M, Fleming S, Mawkin M, et al. Anaesthesia in austere environments: Literature review and considerations for future space exploration missions. Npj Microgravity 2018;4(1):1–11.
- Rachel Homer J, Elwood T, Peterson D, et al. Risk factors for adverse events in children with colds emerging from anesthesia: A logistic regression. PaediatrAnaesth 2007;17(2):154-61.
- Becke K. Anesthesia in children with a cold. CurrOpinAnaesthesiol 2012;25(3):333-9.
- Tait AR, Malviya S. Anesthesia for the child with an upper respiratory tract infection: Still a dilemma? AnesthAnalg 2005;100(1):59-65.
- Gupta A, Saha U. Spinal anesthesia in children: A review. J Anaesthesiol Clin Pharmacol 2014;30(1):10–8.
- Frumiento C, Abajian JC, Vane DW. Spinal anesthesia for preterm infants undergoing inguinal hernia repair. Arch Surg 2000;135:445–51.
- Bhatia U, Abraham S, Panchal M et al. Intrathecal bupivacaine versus bupivacaine and clonidine in pediatrics: A double-blind controlled study. Ain-Shams J Anesthesiol 2022; 14(77): 1-8.
- Bosenberg A. Benefits of regional anesthesia in children. PaediatrAnesth 2012; 22: 10–18.
- Hermanns H, Stevens MF, Werdehausen R, et al. Sedation during spinal anaesthesia in infants. Br J Anaesth 2006;97:380–4.
- Williams RK, Adams DC, Aladjem EV, et al. The safety and efficacy of spinal anesthesia for surgery in infants: The vermont infant spinal registry. AnesthAnalg 2006;102(1):67–71.
- Seyedhejazi M, Moghadam A, Sharabiani BA, et al. Success rates and complications of awake caudal versus spinal block in preterm infants undergoing inguinal hernia repair: A prospective study. Saudi J Anaesth 2015;9(4):348–52.
- Dohms K, Hein M, Rossaint R, et al. Inguinal hernia repair in preterm neonates: Is there evidence that spinal or general anesthesia is the better option regarding intraoperative and postoperative complications? A systematic review and meta-analysis. BMJ Open 2019;9(10):e028728.
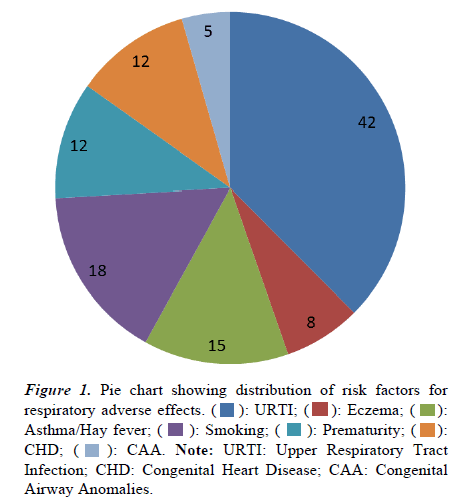
 ): URTI; (
): URTI; ( ): Eczema; (
): Eczema; ( ): Asthma/Hay fever; (
): Asthma/Hay fever; ( ): Smoking; (
): Smoking; ( ): Prematurity; (
): Prematurity; ( ): CHD; (
): CHD; ( ): CAA. Note: URTI: Upper Respiratory Tract Infection; CHD: Congenital Heart Disease; CAA: Congenital Airway Anomalies.
): CAA. Note: URTI: Upper Respiratory Tract Infection; CHD: Congenital Heart Disease; CAA: Congenital Airway Anomalies.