Research Article - Current Pediatric Research (2017) Volume 21, Issue 3
Spectrum of congenital heart disease in children with Down syndrome in Ile-Ife, Nigeria.
John Akintunde Okeniyi1*, Uvie Ufuoma Onakpoya2, Ibitoye Samuel3, Oluwakemi Tolu Adegoke4 and Julia Okolugbo5
1Department of Paediatrics and Child Health, Obafemi Awolowo University, Ile-Ife, Nigeria.
2Cardiothoracic Surgery Unit, Department of Surgery, Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Nigeria
3Department of Paediatrics, Federal Medical Centre, Owo, Nigeria.
4Paediatric Cardiology Unit, Department of Paediatrics, Obafemi Awolowo University Teaching Hospitals Complex, Ile- Ife, Nigeria.
5Department of Paediatrics, Federal Teaching Hospital, Ido-Ekiti, Nigeria.
- *Corresponding Author:
- John Akintunde Okeniyi
BSC, MBChB, FWACP
Department of Paediatrics and Child Health
Obafemi Awolowo University
Ile-Ife, Osun State 220005
Nigeria.
Tel: +2348057647947; +2347087890023
E-mail: jaookeniyi@gmail.com
Accepted date: May 29, 2017
Abstract
Background: Congenital heart disease is a significant morbidity and determinant of mortality in persons with Down syndrome, the most prevalent human chromosomal anomaly. Yet, dearth of literature persists about its spectrum in Nigerian children. Objective: To determine the spectrum and outcome of congenital heart disease among children with Down syndrome in the Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria. Methods: A prospective study of all children diagnosed with Down syndrome seen over a 21 month period. Data obtained and analysed included age, sex, parental ages, birth order, anthropometry, echocardiography diagnoses, complications, treatments and outcomes. Results: Of the 70 children with Down syndrome, 53 (75.7%) had congenital heart disease of which 24 (45.3%) had solitary lesions with atrio-ventricular canal defect being the most common while 29 (54.7%) had multiple lesions of which the combination of ventricular septal defect with atrial septal defect was the most common. However, when all individual lesions were summed together, the most prevalent was atrial septal defect. The mean maternal and paternal ages were 31.4 and 41.2 years respectively. Over half were 1st or 2nd born. Higher proportion of those with congenital heart disease than those without were undernourished (67.9% versus 35.3%; p=0.035). Only 4 (7.5%) have had definitive corrective interventions, 26.4% developed pulmonary arterial hypertension and 18.9% had left ventricular dysfunction. Conclusion: The occurrence of congenital heart disease in these Nigerian children with Down syndrome is high the pattern being comparable with that in the literature with atrio-ventricular canal defect and other septal defects being most common. Presentation is late and the level of definitive care is poor. Routine and early cardiologic evaluation of all children suspected with Down syndrome is advocated.
Keywords
Anomaly, Down syndrome, Cardiac defects, Cardiac malformations, Congenital heart disease, Trisomy 21.
Abbreviations
ASD: Atrial Septal Defect; AVCD: Atrioventricular Septal Defect; CHD: Congenital Heart Disease; DORV: Double Outlet Right Ventricle; DS: Down Syndrome; LSVC: Left Superior Vena Cava; OAUTHC Obafemi Awolowo University Teaching Hospitals Complex,, Ile-Ife, Nigeria; PAH: Pulmonary Arterial Hypertension; PDA: Patent Ductus Arteriosus; PS: Pulmonary Stenosis; SD: Standard Deviation; TGA: Transposition of the Great Arteries; TOF: Tetralogy of Fallot; VSD: Ventricular Septal Defect
Introduction
Down syndrome (DS) or Trisomy 21, the most common human chromosome disorder predisposes affected individuals to a myriad of multi-systemic manifestations and mental sub-normalcy [1-4]. Globally, its prevalence is about 9.6 per 10,000 live births with incidence reportedly varying between 1:650-700 but reportedly lower in southwest Nigeria (1:865 live births) [5]. Affected children exhibit characteristic suggestive features although chromosomal analysis of peripheral white blood cells confirms the diagnosis of Trisomy 21 [6]. They manifest a characteristic facial dysmorphism, various congenital anomalies, including cardiac or gastrointestinal defects, variable degrees of intellectual disability, hypotonia and joint laxity [7].
Congenital heart disease (CHD) occurs in 40-60% of children with DS [7-9]. The most common types reported being atrio-ventricular canal defects (AVCD), ventricular septal defects (VSD), patent ductus arteriosus (PDA), atrial septal defects (ASD) and Tetralogy of Fallot (TOF) in decreasing order [7,10]. Early diagnosis of CHD in these children has been advocated as they tend to readily develop irreversible pulmonary vascular disease earlier than children without DS who have similar cardiac lesions [7]. Children with DS who do not have CHD have much better outcomes and early corrective surgery for those with CHD greatly improves life expectancy [2,3,7]. It is noteworthy that pericardial effusion is reportedly common in children with DS [11].
There are few reports concerning CHD among Nigerian children with DS [5,12]. Reports by Ekure et al. [13] suggest AVCD and VSD as the leading defects in Lagos. Otaigbe et al. [14] reported that PDA and VSD are the most common lesions seen in Port Harcourt. Thus in undertaking this study, we aimed to establish the pattern, frequency and presentation of congenital heart diseases in children with Down syndrome in our clinical setting in Ile- Ife, south west Nigeria.
Methods
This was a prospective hospital-based observational and descriptive study conducted at the Obafemi Awolowo University Teaching Hospitals Complex (OAUTHC), Ile- Ife. The OAUTHC is the major tertiary health care centre that serves children in Ile-Ife and the adjoining rural communities of south-western Nigeria. From consecutive children referred to the paediatric cardiology unit of the hospital via the various paediatric clinics, cardio-thoracic surgical clinic, the children’s emergency room and the general paediatric wards, those with Down syndrome were recruited. Consent to participate was obtained from each parent and the study was approved by the Department of Paediatrics, OAUTHC, Ile-Ife prior to commencement. The study period was the 21 months between 01 August 2015 and 31 March 2017. Each subject underwent routine clinical evaluation. The transthoracic echocardiography was done by the consultant paediatric cardiologist using the Esaote™ MyLab30 Gold® Cardiovascular machine serial number 08538. Data obtained included indications for referral to the paediatric cardiology unit, age, sex, birth weights, birth order and parental ages. The children’s weights, lengths or heights and body surface area were obtained and cross-referenced with standards to determine their nutritional status. The echocardiography findings, complications, treatments and outcomes were also documented. Data were analysed using the Windows version of the Computer Programme for Epidemiologists software (WinPEPI version 11.65) using simple descriptive statistics such as ratios, proportions and percentages [15]. Comparison of means was by use of the Student’s t-test while proportions were compared using the Chi-squared test with Yate’s correction applied whenever necessary: statistical significance was defined for p ≤ 0.05.
| Male | Female | Total | χ2 | p value | ||
|---|---|---|---|---|---|---|
| Down syndrome | CHD | 21 (39.6) | 32 (60.4) | 53 (75.7) | 0.932 | 0.334 |
| No CHD | 9 (52.9) | 8 (47.1) | 17 (24.3) | |||
| Other syndromes | CHD | 3 (50.0) | 3 (50.0) | 6 (30.0) | 0.556 | 0.456 |
| No CHD | 11 (78.6) | 3 (21.4) | 14 (70.0) | |||
| Non-syndromic | CHD | 72 (48.3) | 77 (51.7) | 149 (25.3) | 0.019 | 0.890 |
| No CHD | 216 (49.0) | 225 (51.0) | 441 (74.7) | |||
| Total | 332 (48.8) | 348 (51.2) | 680 (100.0) | |||
Table 1. Gender distribution of the children
Results
During the period of study, 680 children were seen by the paediatric cardiology unit of the hospital and of these, 70 (10.3%) had DS, 20 (2.9%) had other syndromes while the remainder 590 (86.8%) were non-syndromic. They all underwent transthoracic echocardiography. A total of 208 children, i.e., 30.6% had CHD. Fifty-three (75.7%) of the children with DS as against 6 (30.0%) of those who had other syndromes and 149 (25.3%) of the 590 nonsyndromic children were found to have CHD. The higher percentage of the children with CHD observed among those with DS relative to the non-syndromic children was very highly significant statistically (χ2Yate’s correction applied=72.662 and p<0.0001). The gender distribution of all children evaluated is detailed in Table 1. There were many more girls than boys in virtually all the subgroups of children as well as among those who had CHD within each subgroup except those with other syndromes. However, none of these observed differences was statistically significant.
Table 2 shows details of the ages of the children with DS, their birth weights, birth order and their parents’ ages. Their ages ranged from 8 weeks to 9 years. None of the children presented in the neonatal period. Comparison of birth weights and ages at presentation reveal that those with CHD had lower birth weights and presented earlier relative to those without CHD. However, these observations lacked statistical significance. The birth orders of children with DS, with or without CHD were similar as over half in both groups were either the first or second born of their parents. The mean ages of mothers and fathers were less than 35 years and 45 years respectively. However, the mean ages of both the mothers and fathers were significantly lower among children with CHD than those without. Indeed, 35 (53.0%) of the 66 mothers who knew their ages were younger than 30 years. Twenty-seven of these younger mothers had children with DS who also had CHD.
| CHD 53 (75.7%) |
No CHD 17 (24.3%) |
Statistical analysis | |
|---|---|---|---|
| Age (months) | t=-0.13, df=25.1, p=0.895 |
||
| 1.Mean (SD) | 11.98 (15.26) | 12.59 (16.78) | |
| 2.Median | 8 | 8 | |
| 3.Standard error | 1.77 | 3.95 | |
| 4.Range | 3-108 | 2-72 | |
| Birth weight (kg) {Mean (SD)} [N*=61] |
2.55 (0.45) [n=49] |
2.75 (0.37) [n=12] |
t=-1.60, df=19.8 p=0.124 |
| Birth order [n (%)] | χ2=1.291, df=3 p=0.731 |
||
| 1.1st born child | 11 (20.8%) | 3 (17.6%) | |
| 2.2nd born child | 16 (30.2%) | 7 (41.2%) | |
| 3.3rd born child | 22 (41.5%) | 5 (29.4%) | |
| 4.≥ 4th born child | 4 (7.5%) | 2 (11.8%) | |
| Maternal age (years) {Mean (SD)} [N*=66] |
31.4 (4.5) [n=50] |
34.6 (3.8) [n=16] |
t=-2.80, df=29.7, p = 0.009 |
| Paternal age (years) {Mean (SD)} [N*=57] |
41.2 (5.3) [n=42] |
44.6 (3.2) [n=15] |
t=-2.92, df=41.3 p=0.006 |
Key: *: Parents could not provide information in some cases.
Table 2. Ages, birth weights, birth order and parental ages of the 70 children with Down syndrome.
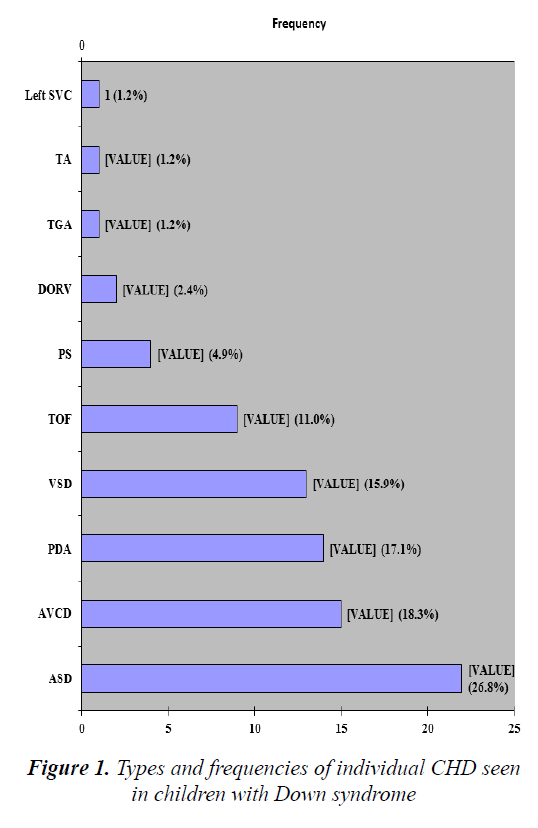
The various reasons for which the children with DS were referred to the paediatric cardiology unit of the hospital are detailed in Table 3. Majority had multiple reasons for consultations ranging from dysmorphism to heart failure. 24 (45.3%) of the 53 children with DS and CHD had solitary lesions of which atrio-ventricular canal defect (AVCD) was the most common. Table 4 provides details of the various solitary and multiple CHD diagnosed among the children with DS. Tetralogy of Fallot was the most common solitary cyanotic CHD. The slight majority (54.7%) had multiple lesions of which the combination of ventricular (VSD) with atrial septal defect (ASD) was most common. However, when all individual observed types of lesions were cumulatively summed together, the most prevalent was ASD, accounting for 22 (26.8%) of the cumulative 82 individual lesions (Figure 1).
| Reason for cardiac referral** | Number(n) | Percentage(%) |
|---|---|---|
| 1.Dysmorphism | 57 | 81.4 |
| 2.Failure to thrive | 38 | 54.3 |
| 3.Murmur | 36 | 51.4 |
| 4.Chest infection | 20 | 28.6 |
| 5.Cyanosis | 16 | 22.9 |
| 6.Heart failure | 13 | 18.6 |
Key: **: Some were referred with multiple reasons.
Table 3.Reasons for cardiac referral of the 70 children with Down syndrome.
| Type of CHD Lesion | Number (percentage) |
|---|---|
| Single lesions | 24 (45.3) |
| 1.Atrioventricular canal defect (AVCD) | 8 (15.1) |
| 2.Tetralogy of Fallot (TOF) | 4 (7.5) |
| 3.Ventricular septal defect (VSD) | 3 (5.7) |
| 4.Ostium secundum Atrial septal defect 5.(ASD) | 3 (5.7) |
| 6.Patent ductus arteriosus (PDA) | 2 (3.8) |
| 7.Pulmonary stenosis (PS) | 2 (3.8) |
| 8.Double outlet right ventricle (DORV) | 1 (1.9) |
| 9.Truncus arteriosus (TA) | 1 (1.9) |
| Multiple lesions | 29 (54.7) |
| 1.VSD/ASD | 8 (15.1) |
| 2.ASD/PDA | 5 (9.4) |
| 3.AVCD/PDA | 4 (7.5) |
| 4.TOF/PDA | 3 (5.7) |
| 5.AVCD/ASD | 3 (5.7) |
| 6.TOF/ASD | 2 (3.8) |
| 7.VSD/PS | 2 (3.8) |
| 8.DORV/TGA | 1 (1.9) |
| 9.ASD/Left Superior Vena Cava (LSVC) | 1 (1.9) |
| Total | 53 (100.0) |
Table 4. Distribution of single and multiple CHD in children with Down syndrome
At presentation, among all 70 children with DS, 42 (60.0%) were undernourished, 36 of whom had CHD and the remaining six were among the 17 children with DS but without CHD. Thus, this higher proportion (67.9%) of children with DS with CHD being undernourished relative to 35.3% among those without CHD was statistically significant (χ2 Yate’s correction applied=4.432 and p=0.035). From among the 53 DS children with CHD, 14 (26.4%) had developed pulmonary arterial hypertension, 10 (18.9%) had left ventricular dysfunction and 10 (18.9%) had varying degrees of pericardial effusions.
Only 4 (7.5%) of the children with DS in contrast with 21 (14.1%) of the non-syndromic children have had definitive corrective interventions of their CHD. 25 (47.2%) have been unable to have definitive treatments because of costs of surgery, 2 (3.8%) declined open heart surgery on religious grounds and 10 (18.9%) opted for faith-based interventions. Ten (18.9%) of the children with DS and CHD have died and another 4 (7.5%) lost to follow-up. The remainder are undergoing palliative care.
Discussion
In this study, 75.7% of children with Down syndrome had congenital heart disease. This is higher than the 40- 60% documented in some literature [7-9]. But similar to the findings in Kano, Northern Nigeria by Asani et al. [12] who reported 77.1% and Shrestha and Shakya [16] who reported 80% from Nepal. In addition to plausible racial and geographic influences, another reason for this discrepancy may be that most of our subjects were referred because they had clinical features suggestive of cardiac defects and not just dysmorphism.
We found solitary cardiac lesions in 45.3% of our subjects with DS with AVCD being the most common lesion similar to the findings of Ekure et al. [13] in Lagos and Asani et al. [12] There are varied reports as to the most prevalent cardiac lesions seen in children with DS with AVCD being the most common [8,17-19]. Mourato et al. [20] and Vilas et al. [21] in Brazil reported ASD while Shrestha and Shakya [16] and Laursen [22] reported VSD. Otaigbe et al. [14] reported PDA to be the most common lesion in Port Harcourt, Nigeria similar to a study in Guatemala [23]. In our study, 54.7% of DS patients with CHD had multiple lesions with the combination of VSD and ASD being the most common. Shrestha and Shakya reported PDA as the most frequently associated lesion followed by ASD among patients with multiple lesions [16]. Granzotti et al. [24] found a significant number of children with Tetralogy of Fallot while other studies have shown low incidence of this lesion [25]. In this study, 16.9% of children with DS had TOF. Presence of multiple defects in these children may worsen their prognosis and predispose them to the development of complications earlier than other non-syndromic children with CHD. This buttresses the need to refer all DS children for routine echocardiographic examination as soon as the diagnosis of DS has been made in them.
The mean age of about 12 months of the children with DS at presentation in the present study illustrates the delay in clinical suspicion of associated congenital heart diseases in these children. In contrast, diagnosis of CHD would have been made earlier in children with DS in developed countries [7]. Absence of foetal diagnosis and early neonatal echocardiographic screening of children with DS in developing countries caused delay in making a definitive diagnosis of CHD in such children. None of the children from this series was diagnosed with CHD in the neonatal period. Other contributors to delay in making the diagnosis of CHD in children with DS may include delivery outside of a health facility, wrong cultural beliefs and poor health seeking behaviour.
More than half of the children with DS in the present study were the first or second born children of their parents. Otaigbe et al. [14] also reported 60% of the children as second born different from previous studies of patients in which they were mainly first born [16,26]. Also a slight majority of the mothers of the children with Down syndrome were younger than 30 years. Similarly, Figueroa et al reported a high proportion of 34% of young mothers of children with DS [26]. The high proportion of young mothers with DS may be explained by greater number of pregnancies occurring in this age group. Contribution of environmental factors, such as abnormalities of folate metabolism, to the aetiopathogenesis of Down syndrome may also partly explain why a significant majority of mothers of patients with DS are in the younger age category in the present study [27]. This has great social implication on the people of Ile-Ife and possibly by inference on Nigerians. There is a need to increase awareness of congenital malformation, especially DS among the population. Foetal screening and early neonatal echocardiographic evaluation of children with congenital malformation should be encouraged and advocated. The mean ages of both mothers and fathers were also significantly lower among children with CHD than those without. An old study by Rowe and Uchida found no apparent difference in the maternal age distribution in cases with and without congenital heart diseases [28]. A more recent study by Khoury and Erickson also did not find a significant difference when he compared the proportion of DS with CHD between women <35 years and those ≥ 35 years [29]. Freeman et al. [8] also reported no relationship between maternal age and congenital heart diseases. Vilas et al. [21] reported that half of the mothers and 89% of the fathers were older than 35 years during pregnancy. However, parental age did not have an influence on the occurrence of congenital heart diseases in other studies [21,30,31].
Our study also showed that DS patients with CHD had lower birth weights and presented earlier than those without. Malnutrition is common in children with DS. Those with congenital heart disease are smaller and lighter than those without or with mild disease [32].
Pulmonary arterial hypertension (PAH) was recorded in 26.4% of the children with DS. Shrestha reported 52.5% having PAH and Mourato recorded 37.5% developing PAH [16,20]. It has been reported that patients with DS develop pulmonary hypertension early when they have left to right shunt lesions. Individuals with DS may have pulmonary arterial hypertension for various reasons such as chronic airway obstruction, abnormal growth of the pulmonary vasculature, alveolar hypoventilation, decreased number of alveoli and thinner pulmonary arterioles [33,34].
21 (14.1%) of the non-syndromic children had corrective surgery compared with only 4 (7.5%) of the children with DS. This is mainly because many parents could not afford the cost of treatment particularly for those patients with complex lesions. Also, many of them had developed complications such as severe PAH. The presence of other health challenges in some of the patients, which the parents had to constantly address, probably also limited the chances of the parents of being financially buoyant enough to pay for the cost of correction of heart defects. Thus, there is need for the establishment of Foundations and Trusts to support the care of the children with DS and their families in this community to brighten their chances of earlier surgical correction of CHD with the likelihood of improved quality of life.
Conclusion
The occurrence of CHD among children with Down syndrome seen in our setting is higher than previously documented in other regions of Nigeria. Majority of these children have multiple lesions with atrial septal and atrioventricular canal defects being the most common. These children present late and the level of definitive care they currently receive is minimal. We advocate for routine and early cardiologic evaluation of all children suspected with Down syndrome.
Acknowledgement
We acknowledge with thanks, the efforts of Prof. Oluwagbemiga Adeodu (MBBS, FWACP) of the Department of Paediatrics and Child Health, Obafemi Awolowo University, Ile-Ife in the review of the manuscript.
Declarations
Availability of Data and Materials
The data will not be shared to respect the privacy of participants.
Authors’ Contributions
Study design: JO.1; Data collection and interpretation: JO1, IS, OA, JO; Manuscript preparation: JO1, UO; Literature search: UO, OA, IS, JO; Final approval: All authors.
Consent for Publication
Consent to publish was obtained from all patients’ parents.
Ethics Approval and Consent to Participate
Sort and obtained from the Department of Paediatrics, OAUTHC, Ile-Ife.
References
- Descartes M, Carroll AJ. Cytogenetics. In: Kleigman RM, Berhman RE, Jenson HB, Stanton BF editors. Nelson Textbook of Pediatrics. 18th Edn. Philadelphia: Saunders 2007: 507-508.
- Kazemi M, Salehi M, Kheirollahi M. Down syndrome: Current status, challenges and future perspectives. Int J Mol Cell Med2016; 5: 125-133.
- Espinola ZN, Soto ME, Romero-Gonzalez A, et al. Prevalence of congenital heart disease and pulmonary hypertension in down's syndrome: An Echocardiographic Study. J Cardiovasc Ultrasound 2015; 23: 72-77.
- Asim A, Kumar A, Muthuswamy S, et al. Down syndrome: An insight of the disease. J Biomed Sci 2015; 22: 41.
- Adeyokunnu AA. The incidence of down syndrome in Nigeria. J Med Genet 1982; 19: 277-279.
- Patterson D. Molecular genetic analysis of down syndrome. Hum Genet 2009; 126: 195-214.
- Ko JM. Genetic syndromes associated with congenital heart disease. Korean Circ J2015; 45: 357-361.
- Freeman SB, Taft LF, Dookey KJ, et al. Population based study of congenital heart disease in Down syndrome. Am J Med Genet 1998; 80: 213-217.
- Wells GL, Baker SE, Finley SC, et al. Congenital heart disease in infants with Down syndrome. South Med J 1994; 87: 724-727.
- Cleves MA, Hobbs CA, Cleves PA, et al. Congenital defects among live born infants with Down syndrome. Birth Defects Res A Clin Mol Teratol 2007; 79: 657-663.
- Concolino D, Pascuzzi A, Pietragalla E, et al. High prevalence of isolated pericardial effusion in down syndrome. Am J Med Genet A 2005; 132A: 331-332.
- Asani M, Aliyu I, Also U. Pattern of congenital heart diseases among children with Down syndrome in Aminu Kano Teaching Hospital, Kano, Nigeria. Niger J Basic Clin Sci 2013; 10: 57-59.
- Ekure EN, Animashaun A, Bastos M, et al. Congenital heart disease associated with identified syndromes and other extra cardiac congenital malformations in children in Lagos. West Afr J Med 2009; 28: 33-37.
- Otaigbe BE, Tabansi PN, Agbedeyi GO. Pattern of congenital heart diseases in children with Down syndrome at the University of Port Harcourt Teaching Hospital, Port Harcourt. Niger J Paed 2012; 39: 164-167.
- Abramson JH. WINPEPI updated: computer programs for epidemiologists and their teaching potential. Epidemiol Perspect Innov 2011; 8: 1.
- Shrestha M, Shakya U. Down syndrome and congenital heart disease: Single centre, prospective study. NJMS 2013; 2: 96-101.
- Nisli K, Oner N, Candon S, et al. Congenital heart disease in children with down syndrome: Turkish experience of 13 years. Acta Cardiol 2008; 63: 585-589.
- Mikkelsen M, Poulsen H, Nielsen KG. Incidence, survival and mortality in Down syndrome in Denmark. Am J Genet Suppl 1990; 7: 75-78.
- Stoll C, Alembik Y, Dott B, Rott M. Epidemiology of down syndrome in 118,265 consecutive births. Am J Med Genet Suppl 1990; 7: 79-83.
- Mourato FA, Villachan LRR, Mattos SDS. Prevalence and profile of congenital heart disease and pulmonary hypertension in Down syndrome in a paediatric cardiology service. Rev Paul Pediatr 2014; 32: 159-163.
- Vilas BLT, Albernaz EP, Costa RG. Prevalence of congenital heart disease in patient with Down syndrome in the municipality of Pelotas, Brazil. J Pediatr (Rio J) 2009; 85: 403-407.
- Laursen HB. Congenital heart disease in Down syndrome. Br Heart J 1976; 38: 32-38.
- Vida VL, Barnoya J, Lairazabal LA, et al. Congenital cardiac disease in children with down syndrome in Guatemala. Cardiol Young 2005; 15: 286-290.
- Granzotti JA, Paneto IL, Amaral FT, et al. Incidence of heart defect in Down syndrome. J Pediatr (Rio J)1995; 71: 28-30.
- Rashid AKMM, Basu B, Rahman MM. Tetralogy of fallot in Down syndrome (Trisomy 21) – An uncommon association. Pak J Med Sci 2009; 25: 698-700.
- De Rubens Figueroa J, Del Pozzo Magana B, Pablos Hach JL, et al. Heart malformations in children with Down syndrome. Rev Esp Cardiol 2003; 56: 894-899.
- Sherman SL, Allen EG, Bean LH, Freeman SB. Epidemiology of Down syndrome. Ment Retard Dev DisDisabil Res Rev2007; 13: 221-227.
- Rowe RD, Uchida IA. Cardiac malformation in Mongolism. Am J Med 1961; 31: 726-735.
- Khoury MI, Erickson JD. Improved ascertainment of cardiovascular malformation in infants with Down syndrome, Atlanta, 1968-1989. Am J Epidemiol 1992; 136: 1457-1464.
- Hayes C, Johnson Z, Thornton L, et al. Ten year survivals of Down syndrome births. Int J Epidemiol 1997; 26: 822-829.
- Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population based registry. Am J Med Genet 1998; 77: 431-438.
- Bravo-Valenzuela NJ, Passaralli ML, Coates MV, et al. Weight and height recovery in children with Down syndrome and congenital heart disease. Rev Bras Cir Cardiovasc 2011; 26: 61-68.
- Chi TPL, Krovetz J. The pulmonary vascular bed in children with Down syndrome. J Pediatr1975; 86: 533-538.
- Banjar HH. Down syndrome and pulmonary arterial hypertension. PVRI Review 2009; 42: 213-216.