Research Article - Journal of Industrial and Environmental Chemistry (2018) Volume 2, Issue 1
Spectrophotometric quantification of germanium (IV) in environmental samples using chemically modified chitosan sorbent and optimized cloud phase extraction method
Islam MI Moustafa1, Zakia AL-Mallah2, Alaa S Amin1*1Chemistry Department, Benha University, Benha, Egypt
2Chemistry Department, Umm AL-Qura University, Makkah, Saudi Arabia
- *Corresponding Author:
- Alaa S Amin
Chemistry Department
Benha University
Benha
Egypt
Tel: +20552350996
E-mail: asamin2005@hotmail.com
Accepted Date: November 20, 2017
Citation: Moustafa IMI, AL-Mallah Z, Amin AS. Spectrophotometric quantification of germanium (IV) in environmental samples using chemically modified chitosan sorbent and optimized cloud phase extraction method. J Ind Environ Chem. 2018;2(1):1-8
Abstract
Biopolymers as chitosan which is one of the emerging sorption material for the removal of metal ions was applied to determine germanium(IV), even at low concentrations. A selective, sensitive and rapid method was described for preconcentrative determination of Ge(IV) using the synthesized 2-amino-4-(m-tolyazo)pyridine-3-ol (ATAP), which was chemically immobilized on chitosan. The sorbent synthesized material was selective to Ge(IV) within a best response time of 10 min. The procedure was selective in presence of other diverse anions and cations. Under the optimum experimental conditions, calibration plots were linear over the concentration range of 2.5 to 75 ng mL−1 of Ge(IV). The obtained results are 100 times lower than by the direct determination of Ge(IV) by FAAS. The developed method is repeatable with a relative standard deviation of 2.55%. The developed sorbent was applied successfully to determine Ge(IV) in environmental samples. Unlike most preconcentration methods, the present enrichment work allowed for a rapid and reliable determination of Ge(IV) in real water and soil samples by the simple, rapid and routinely available spectrophotometric technique.
Keywords
Germanium (IV) determination, Chitosan, Preconcentration, Spectrophotometry, Environmental samples
Introduction
Germanium is present in nature dispersed in ore deposits, mainly as soluble methylgermanium species and germanate anions [1,2]. It is an element widely used in, e.g., as a catalyst in production of plastics or semiconductor production [3]. Germanium determination and its concentration on ultra-trace levels has been pursued for many years [3,4]. Enrichment (also named preconcentration) is a generic term for different processes employed to increase the level of a desired element to be suitable for further processing, e.g., its determination, increases the sensitivity by several orders of magnitude, facilitates calibration, preconcentration improves the analytical detection limit, and enhances the accuracy of the results. Some preconcentration procedures, e.g., sorption [5–10], liquid-liquid extraction [11–15], coprecipitation [16,17], and cloud point method [18], is employed in combination with spectroscopic and spectrophotometric detection methods [11,19-33] to determine Ge(IV) ions. The germanium preconcentration by sorption offers various advantages over other methods of concentration (e.g., liquid-liquid extraction), such as experimental convenience, usage of less toxic materials and low cost. To date, there are a few studies related to preconcentration Ge(IV) ions on a solid substrate, for instance on Kelex-100 [11], nanometer sized TiO2/SiO2 [3], TiO2 [2,19], active carbon [34], cellulose [9], chitosan chelating resin [5,9,11], Sephadex gel [6], anionic resin (IRA-900) [31], goethite [34] and mercapto modified silica gel [8]. Therefore, the described sorbents have one or more of the following disadvantages: low rate of sorption [3,27,29], relatively low selectivity, low sorption capacity [34], or high cost of sorbent [7,34,35]. A new inexpensive sorbent with best analytical parameters for at least some of the above mentioned points constitutes an important task.
Several analytical reagents have already been applied for the spectrophotometric determination of Ge(IV) [11,19-33], whereas, the analytical parameters for them are rather moderate. In fact, none of the reagents described have good integrate analytical parameters (large scale for determination, low detection limits, low cost and selectivity). Therefore, introduce a new effective reagent; 2-amino-4-(m-tolyazo)pyridine-3-ol (ATAP), for easy and straight forward determination of Ge(VI) was decided.
Recently, to treat low concentration of heavy metals from environmental samples chitosan was used due to its high adsorptive capacity when compared to other adsorbents [25,26]. In this study, a rapid selective and sensitive procedure is investigated to preconcentrative determination of Ge(IV) applying the synthesized 2-amino-4-(m-tolyazo)pyridine-3- ol (ATAP), which was chemically immobilized on chitosan. Therefore, the goal of this study is to develop a new routine procedure for the effective preconcentration of Ge(IV) combined with its spectrophotometric determination.
Methodology
Apparatus
Atomic absorption spectrometer model 6300 (AAS), Shimadzu (Japan), was used for measurements with flame of N2O-C2H2 and the instrument settings were a adjusted according to the manufacturer’s recommendations. IR spectrometer (Thermo- Nicolet FT-IR, Nicolet IR-200, USA) was used for the analysis of functional groups in the synthesized reagent. An Orion research model 601 A/digital ionalyzer pH meter was used for checking the pH of solutions. A Perkin-Elmer Lambda 12 UV/ Vis spectrometer was used for recording absorbance spectra with 5.0 mm quartz cell. A centrifuge with 25-mL calibrated centrifuge tubes (Superior, Germany) was used to accelerate the phase separation process.
Special chemical preparations
All the reagents used in this work were of analytical grade and all solutions were prepared in polypropylene volumetric flasks. A 1.00 × 10–2 M solution of Ge(IV) was prepared by dissolving metallic germanium (99.99%) as described [36] and Ge(IV) concentration was determined by atomic absorption spectroscopy [37]. The stock solutions of the various metal ions (mg L–1) were prepared with their nitrate or chloride salts (≥ 99.99%) and used to illustrate the possible effects of interfering ions. Doubly distilled water was used throughout the experiments.
Solutions of alkali metal salts (1.0%) and various metal salts (0.1%) (Sigma, St Louis, MO, USA) were used to study the interference of anions and cations, respectively. Acetate buffer solutions (HOAc–NH4OAc buffer) of pH 2.75–5.61 were prepared as recommended [38].
Synthesis of 2-amino-4-(m-tolyazo)pyridine-3-ol
2-Amino-4-(m-tolyazo)pyridine-3-ol (ATAP) (Figure 1) was prepared according to way used for preparing azo dye derivative of aromatic amine. 0.01 mole of m-toludene was converted to the hydrochloric form by adding the least amount of 1:1 HCl then diluting with water and cooling at –2.0°C. A cooled solution of NaNO2 (0.01 mole) was added gradually with continuous stirring to the amine salt. The resulting diazonium salt solution was allowed to stand in ice bath for 15 min with stirring at –2.0°C and then added gradually to a solution of 0.01 mole of 2-amino-3-hydroxypyridine dissolved in 10% NaOH which cooled at –2.0°C. The resulting solution was allowed to stand for 15 min with constant stirring until the azo dye completely formed. The obtained azo was filtered off, dried and recrystallized in ethanol. The purity of the resulting azo dye was checked by measuring the melting point constancy. The chemical structure was detected by melting point, elemental analysis (C, H, N), IR and 1H-NMR spectra. The separated azo has the following structural formula: A 5 × 10−3 M solution of the reagent was prepared by dissolving an appropriate amount of reagent in 10 mL ethanol and completed to the mark in 100 mL calibrated flask.
General procedure
To determine concentration of Ge(VI), standard solutions were adjusted to pH=3.5 ± 0.1 by adding 3.0 mL of acetic acid acetate buffer. 0.8 mL of 5 × 10−3 M ATAP solution, and 3.0 mL of 5.0% Triton X-100 solution (Sigma, St Louis, MO, USA) was added and allowed to stand for 5.0 min at room temperature. Then 3.0 mL of 0.3 M KCl solution was added. An amount of 40 mg of chitosan was added to the above solution and the mixture was shacked for 5.0 min and made up to the mark in 25 mL measuring flask with doubly distilled water. Separation was accelerated by centrifugation for 5.0 min at 3800 rpm, then the aqueous phase could be isolated by overturning the tube. The surfactant-rich phase was dissolved in 0.1 mL of acetonitrile, and transferred into a 5.0 mm quartz cell. The absorbance of the solution was measured at 547 nm against a blank solution prepared in the same way but without Ge(IV) ions.
Procedure for soil analysis
Soil samples were collected from industrial sites of Shoubra and Quesna cities. The soil samples were collected and air dried at 70°C in the laboratory oven and then grinded to fine powder and sieved through 0.25 mm nylon mesh. A 10 mL concentrated HCl and 3.0 mL concentrated HNO3 were added to one gram of soil sample, and kept for overnight [39]. After digestion and filtration, the solution was subjected to separation by following the general procedure described above.
Application to real samples
The developed method was applied to quantify Ge(IV) ions in the real water and soil samples collected in and around industrial sites of Shoubra and Quesna. The general procedure described above was applied to 20 mL of water sample (tap water/ground water) and acid digested soil sample was followed.
Interference study
The interference of foreign ions like Cl−, F−, SO42−, PO43−, Na+, Ca2+, Zn2+, Fe3+, Fe2+, Cu2+, Cr3+, Mn2+, CO2+ and Ni2+ was studied by equilibrating the fixed amount of Ge4+ along with the reagent sorbent solution at pH=3.5 and later the determinations was made using the general procedure described above.
Results and Discussion
Reaction conditions
The reaction conditions were investigated with 40 ng mL–1 solution of Ge(IV). Sorption was carried out in different buffer media, and other variables were kept constant. It was found that Ge–complex was quantitatively sorbed on chitosan in an acetate buffer solution of pH 3.5. Addition of 2.5–3.5 mL of a pH 3.5 solution did not affect the CPE of Ge–complex and the use of 3.0 mL is recommended.
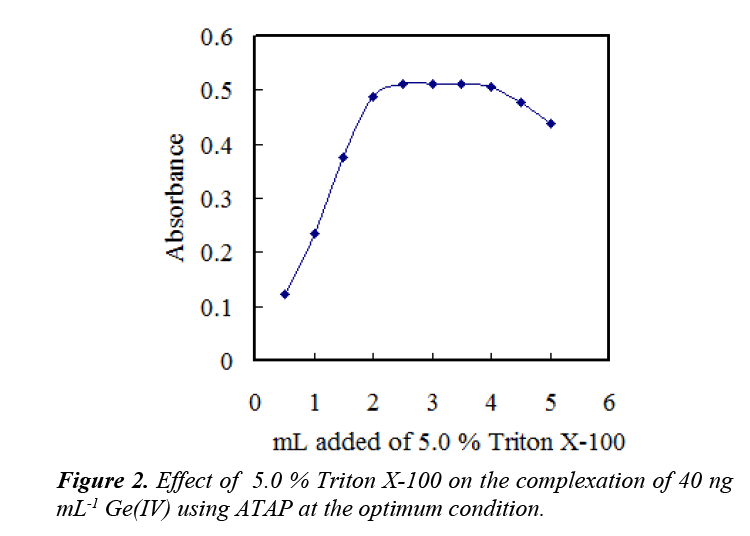
The effects of surfactants on the Ge–ATAP system were studied. The results indicated that, in the presence of anionic or cationic surfactants, the Ge–ATAP chromogenic system gives a low absorption, whereas in the presence of nonionic surfactants, the absorption of the chromogenic system increases markedly. Various nonionic surfactants enhance the absorbance in the following sequence:
Triton X-100 > Triton X-114 > Tween-20 > Tween-60> Tween-80> emulsifier-OP
The Triton X-100 was the optimum one, and the use of 2.5–3.5 mL of 5.0% Triton X-100 solution gave a constant and maximum absorbance value (Figure 2). Consequently, the use of 3.0 mL was recommended.
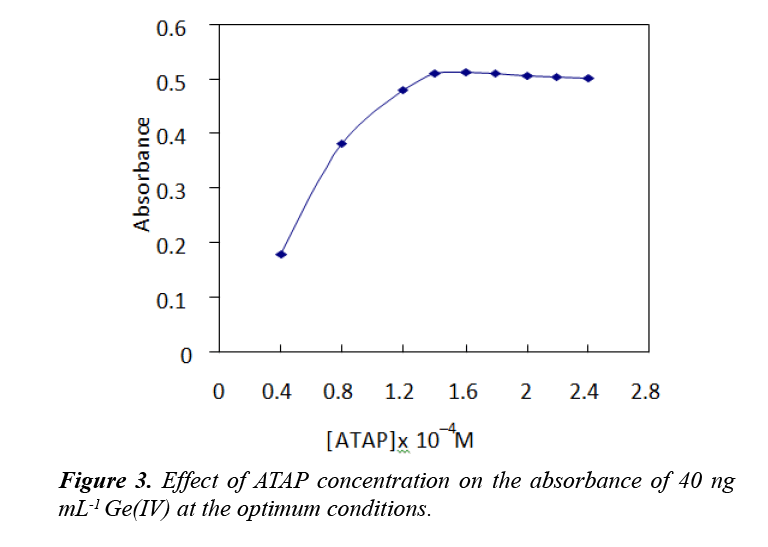
For up to 40 ng of Ge(IV), The effect of ATAP concentration on the extraction and quantification of Ge(IV) was illustrated in the range of (0.2–1.4 mL) of 5 × 10−3 M. The sensitivity of the procedure was increased by increasing the ATAP concentration up to 1.4 × 10−4 M and remained constant at higher concentrations. Therefore, 1.6 × 10−4 M ATAP was applied in all further work. The results are represented in Figure 3. The slight decrease in absorbance after 1.6 ×10−4 M ATAP by about 3.0% is likely to be due to the concentration of uncomplexed ATAP in the surfactant rich phase being increased significantly, so free ATAP competes with the complexes for extraction to the surfactant rich phase.
The volume of the aqueous phase was changed in the range of 2.0–100 mL under the optimum experimental conditions, keeping the other variables constant. It was observed that the highest absorbance value was almost constant up to 25 mL. However, for convenience, all the experiments were carried out with 25 mL of the aqueous phase.
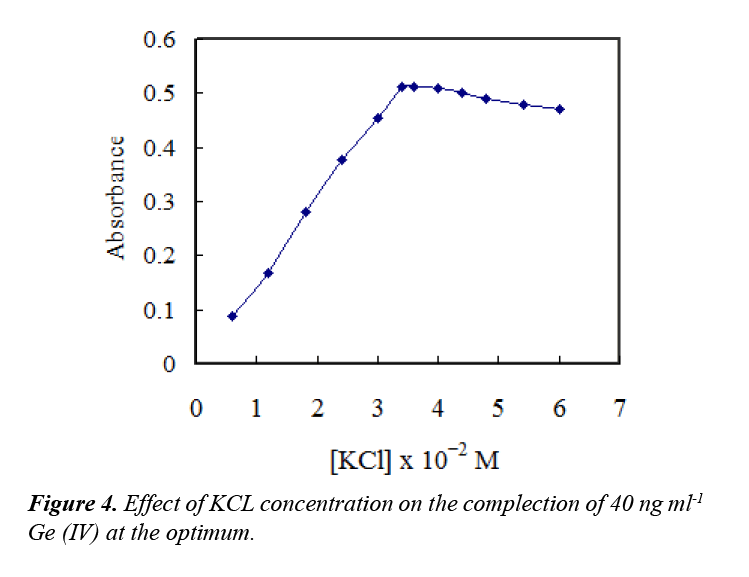
Addition of salts can cause cationic surfactant solutions to separate into two phases: immiscible surfactant-rich and surfactant-poor phases. Therefore, different concentrations of potassium chloride were added to prompt micellar growth and extraction of the formed complex. The effect of chloride concentration was investigated in the range of (0.5–5.0 mL) of 0.3 M. The results indicated that addition of 3.4 × 10−2 M chloride was sufficiently for maximum extraction of the complex and the absorbance remained constant at higher concentrations, as exhibited in Figure 4.
A concentration of 3.6 ×10−2 M chloride was selected for further work. The effect of time on the reaction and also on the CPE procedure was investigated. The results showed that the complex formation of Ge–ATAP was completed in 5.0 min, and 5.0 min centrifugation at 3800 rpm was found to be enough for complete CPE.
The sensitivity and selectivity can be increased by sorbed the formed complex on biopolymer chitosan. Chemically modified chitosan sorbent would be modified the method to be more sensitive and selective. Different weight of chitosan sorbent was tested ranging from 10 to 70 mg. A 40 mg of chitosan gave the highest absorbance value in addition to smallest volume of acetonitrile is used for CPE. Without chitosan, CPE take 2.5 mL of acetonitrile with half absorbance value obtained on using chitosan sorbent, whereas 0.1 mL of acetonitrile was sufficient to dissolve the surfactant-rich phase. Hence using 40 mg chitosan was sufficient for the effective preconcentration. Therefore, a preconcentration factor of 250 was archived using chitosan, whereas it reach 10 only without chitosan.
Stoichiometric ratio
The nature of the complex was illustrated at the optimum experimental conditions described above using the molar ratio and continuous variation methods. The plot of absorbance versus the molar ratio of ATAP to Ge(IV), obtained by varying the ATAP concentration, showed inflection at a molar ratio of 2.0, indicating the presence of two ATAP molecules in the formed complex. Moreover, the Job method showed a ratio of ATAP to Ge(IV)=2.0. Consequently, the results indicated that the stoichiometric ratio was (2 : 1) [ATAP : Ge]. For the ternary complex with Triton X-100, the obtained results implied that a 1 : 1 complex is formed between the [(ATAP)2Ge] complex and Triton X-100. Consequently, the results indicated that the stoichiometric ratio was 2 : 1 : 1 [(ATAP)2Ge][Triton X-100], as shown in the following equations. Using Harvey and Manning equation applying the data obtained from the above two methods, the calculated conditional formation constant (log K), was found to be 3.87, whereas the true constant was 3.65.
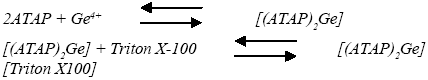
Stability of the chromogenic system
The absorbance reaches its maximum simultaneously at room temperature after mixing the components, and remains stable for 3.0 h in aqueous solution. After extracted into acetonitrile, the complex was stable for at least 12 h.
Effect of foreign ions
The effect of the interfering species upon the sorption was investigated using the proposed procedure at optimized conditions applying 40 ng of Ge(IV). The tolerance limit was set as the concentration of the diverse ion required to cause ± 5.0% error in the determination of Ge(IV). The tolerance limit (error < 5.0%) is recorded in Table 1. The results showed that alkaline and earth-alkaline metals, Mn2+, Cd2+, Zn2+, Ni2+, CO2+, Pb2+, In3+, Bi3+, Al3+, Ga3+, Sn4+, Zr4+, Ti4+, and Hf4+, as well as anions had no significant effect on the separation and determination of Ge(IV) under the recommended experimental conditions, and the most serious interference arose from Cu2+ and Fe3+ (Table 1). However, the interference can be completely eliminated by masking with 0.5 mL of 0.01 M oxalic acid or citric acid. The above results also clearly demonstrate that the described herein sorbents show much better selectivity for the Ge(IV) ion in comparison to the previously described ones, especially in view of such interfering ions as CO2+, Mn2+, Ni2+, Zn2+, Sn4+ and PO43– [2,3,29,30].
Table 1. Separation of Ge(IV) in the presence of different diverse ions.
| Ion | Added as | Concentration (μg mL–1) | Recovery (%) Ge(IV) |
|---|---|---|---|
| Na+ | NaCl | 17000 | 98.2 |
| K+ | KCl | 14000 | 97.5 |
| Mg2+ | MgCl2 | 12000 | 98.2 |
| Ca2+ | CaCl2 | 10000 | 98.9 |
| Ba2+ | BaCO3 | 8500 | 97 |
| Mn2+ | Mn(NO3)2 | 6500 | 96.8 |
| Cd2+ | Cd(NO3)2 | 3500 | 96.7 |
| Ni2+ | Ni(NO3)2 | 2500 | 96.8 |
| Zn2+ | Zn(NO3)2 | 1750 | 96.5 |
| CO2+ | Co(NO3)2 | 1400 | 96.2 |
| Pb2+ | Pb(NO3)2 | 1000 | 97.6 |
| Hg2+ | HgCl2 | 750 | 99 |
| Ag+ | AgNO3 | 600 | 98.5 |
| Ti4+ | Ti(SO4)2 | 500 | 98.6 |
| Pb2+ | Pb(NO3)2 | 400 | 99.5 |
| Al3+ | Al(NO3)3 | 300 | 98.3 |
| In3+ | In(NO3)3 | 250 | 96.5 |
| Ga3+ | Ga(NO3)3 | 225 | 94.2 |
| Sn4+ | Sn(SO4)2 | 200 | 97.3 |
| Zr4+ | Zr(SO4)2 | 175 | 95.9 |
| Hf4+ | Hf(SO4)2 | 150 | 96.5 |
| Bi3+ | Bi(NO3)3 | 100 | 97.8 |
| Cu2+* | Cu(NO3)2 | 50 | 97.2 |
| Fe3+* | Fe(NO3)3 | 25 | 96.2 |
Note:*masked with 0.5 mL of 0.01 M oxalic or citric acid
Calibration curve and sensitivity
The calibration curve indicated that the system obeys Beer’s law in the concentration range of 0.5–75 ng Ge(IV) per mL in the measured solution. For more accurate results, the Ringbom optimum concentration range was found to be 2.0–70 ng Ge(IV) per mL in the measured solution. These values are 100 times lower than by the direct determination of germanium by AAS. The linear regression equation obtained was A=12.8C (μg mL–1) + 0.0053 (r=0.9992). The molar absorptivity was calculated to be 9.30 × 105 L moL–1 cm–1 at 547 nm, whereas the Sandell sensitivity was found to be 0.078 ng cm−2 (Table 2). The standard deviations of the absorbance measurements were calculated from a series of 13 blank solutions. The limits of detection (K=3) and of quantification (K=10) of the method were established [40] and recorded in Table 2, according to the IUPAC definitions (C1=KSo/s where C1 is the limit of detection, So is the standard error of blank, s is the slope of the standard curve and K is the constant related to the confidence interval. The relative standard deviation was 2.25% obtained from a series of 10 standards each containing 40 ng mL–1 of Ge (IV).
Table 2. Analytical features of the proposed method.
| Parameters | CPE without chitosan | Using chitosan |
|---|---|---|
| Amount of acetonitrile | 2.5 | 0.1 |
| pH | 3.5 | 3.5 |
| Optimum [ATAP] M | 1.6 × 10−4 | 1.6 × 10−4 |
| Reaction time (min) | 20 | 5 |
| Stirring time (min) | 10 | 5 |
| Beer’s range (ng mL–1) | 500 - 8500 | 0.5 -75 |
| Ringbom range (ng mL–1) | 1000 - 8000 | 2.0- 70 |
| Molar absorptivity (L mol–1 cm–1) | 2.28 × 104 | 9.30 × 105 |
| Sandell sensitivity (ng cm–2) | 25.2 | 0.078 |
| Regression equation | ||
| Slope (µg mL–1) | 0.314 | 12.8 |
| Intercept | -0.014 | 0.05 |
| Correlation coefficient (r) | 0.9976 | 0.9992 |
| RSD a (%) | 3.1 | 2.25 |
| Detection limits (ng mL) | 130 | 0.17 |
| Quantification limits (ng mL–1) | 440 | 0.5 |
| enhancement factor | 10 | 250 |
| Improvement factor | 12.5 | 450 |
Because the amount of Ge (IV) in 25 mL of the sample solution was measured, and after preconcentration by CPE the final volume is 2.5 mL, the maximum preconcentration factor of the solution is 10. On using modified sorbent chitosan, the preconcentration factor is increase to 250. The improvement factor, defined as the ratio of the slope of the calibration graph for the CPE method without and with chitosan to that of the calibration graph in aqueous media (before applying the CPE method), for Ge(IV) was 12.5 and 450, respectively.
A comparison of the proposed procedure with the previously reported procedures for preconcentration and spectrophotometric determination of Ge(IV)9–33 (Table 3) indicates that the proposed procedure is faster and simpler than the existing procedures and that it provides a lower limit of detection. Although the procedure [32] using modified copolymer styrene-maleic anhydride, (bis(2,3,4- trihydroxyphenylazo)benzidine in the presence of heterocyclic amines) with spectrophotometry has the same selectivity, the proposed procedure has lower detection limits, in addition to lower range of determination. The proposed procedure has more advantages through the sensitivity and interference point of view. To the best of our knowledge, this is the first report of using ATAP as chromophoric reagent for preconcentration and determination of Ge(VI).
Table 3. Survey of spectrometric methods applied for the determination of germanium.
| Spectrophotometric reagent | LOD µg mL–1 | Beer’s law µg mL–1 | Selectivity | Analyzed sample | Ref. |
|---|---|---|---|---|---|
| o-Chlorophenylfluoronea | 0.0– 12 | 125 | Ni(II), Co(II), Sn(II), Fe(II), Zn(II), Mn(II), Pt(IV), Cr(III), W(III), Al(III), V(V), Ti(IV), La(III), Au(III), In(III), Mo(IV), Zr(IV), Sb(III), Ag(I), | Water | [19] |
| 9-(o-Chlorophenyl)-2,6,7-trihydroxyxanthen-3-one in the presence of cetyltrimethylammonium bromidea | 0.0– 200 | Ba(II), Pb(II), Ga(III), Sb(III), V(V), Cr(VI), W(VI), Mo(VI) | Minerals and Ores | [20] | |
| polysulfone membrane filter- Ge(IV)-9-phenyl-3-fluorone complex | 2.1 | Up to 7.0 | Al(III), Fe(III), Si(IV), Sn(IV), | soil and water | [21] |
| Phenylfluorone and zephiraminea | 0 – 0.10 | 47.5 | Sb(III), Sn(II, IV), W(VI), Mo(VI), Ta(V), Nb(V) | Hot spring water and ground water | [7] |
| Nano-sized TiO2 c | 43 | Sr(II), Zn(II), PO43–, F– | Water | [3] | |
| Catechol violet and cetyltrimethylammonium bromidea | 0.1 – 1.0 | Sb(III), Fe(III), Bi(III), Sn(IV), V(V), Cr(VI), Mo(VI), | |||
| o-sulfophenylfluorone (SPF) and cetyltrimethylammonium chloride (CTAC). | 0.0026 | 0.007-0.40 | Sb(III), Sb(V), Sn(II), Sn(IV), Mo(V1), Ti(1V) | human urine | [22] |
| Precipitation with Fe(OH)3 and determination with trimethoxylphenylfluoronea | 0 – 0.24 | 21 | Sr(II), Pb(II), Zr(IV), Ti(IV), Mo(VI) | Foods | [23] |
| Preconcentration on an organic solvent-soluble membrane and determination with o-nitrophenyl-fiuorone in presence of sodium dodecyl sulfate a | 0.02 – 0.36 | 0.0– 40 | Pb(II), Mo(VI) | Chinese herb, Natural-, drinking- waters, urine sample | [15] |
| Nano-TiO2 (salicyl fluorone in the presence of cetyltrimethylammonium bromide)a | 0 – 0.24 | 0.072 | Al(III), Fe(III), Si(IV), Sn(IV), | Water and certified reference material (GBW07311) | [2] |
| Preconcentration/separation procedure (spectrophotometric reagent) Pyrogallolb | 0 – 18 | 12 × 90 | As(III), Sn(IV) | Ore | [24] |
| TiO2 nanoparticles (salicyl fluorone in the presence of cetyltrimethylammonium bromide)a | Li(I), Cu(II), Ba(II), Cd(II), Sr(II), Co(II), Ni(II), Se(IV), Si(IV) | [27] | |||
| Coprecipitation of germanium in the presence of Mg2+, Ga3+, Ca2+ and HCO3– e | 0.006 | Sea-, surface- and ground-waters | [17] | ||
| (Methybenzeneazosalicylfluorone) using ultrasound-assisted leaching a | 0.0– 0.72 | 2.75 | Cr(III), Hg(II), Ti(IV), U(VI), As(III), Bi(III), Se(VI), Te(VI), Be(II), Pt(IV), Pt(II), Pd(II), Ru(IV), Ir(III), Os(VI), Au(III) | Certified reference Materials(GBW 07401 and GBW07402) | [28] |
| Mercapto-modified silica geld | 0.01 – 0.20 | 0.813 | Co(II), Cu(II), Ni(II) | [8] | |
| Kelex-100, [7-(4-ethyl-1-methyloctyl)-8-hydroxyquinoline] functional sol gelb | As(II), Sb(III), Zn(II), Ni(II) | Water | [29] | ||
| Cloud point methodology, triton X-114 d | 10 – 300 | 0.59 | As, Te, Sb | Tap and drinking water | [18] |
| Precipitation with Fe(OH)3 and determination | 0 – 0.24 | 21 | Sr(II), Pb(II), Zr(IV), | Foods | [23] |
| with trimethoxylphenylfluorone a | Ti(IV), Mo(VI) | ||||
| Chitosan functionalized with di-2-propanolamine g | 0 – 0.002 | 50 | Tap-, river-, and seawater | [30] | |
| The separation was performed by an isocratic elution h | 0.05 – 5.0 | Tonic oral liquids | [31] | ||
| Anionic resin (IRA-900), catecholi | Fly ash | [32] | |||
| Modified copolymer styrene-maleic anhydride,(bis(2,3,4-trihydroxyphenylazo) benzidine in the presence of heterocyclic amines) | 0.012 – 0.182 | 0 - 90 | Cu(II), Fe(III) | Seawater and water obtained after oil pumping | [33] |
| ATAP Using chitosan | 0.0017 | 0.005 – 0.075 | Cu(II) and Fe(III) | seawater and water obtained after oil pumping | This work |
Note: LOD: Limit of detection; Detection technique: a: spectrophotometery; b: adsorptive stripping voltammetric; c: graphite furnace atomic absorption spectrometry; d: hydride generation flame atomic absorption spectrometry; e: hydride generation-atomic emission spectrometry; f: flow injection hydride generation atomic fluorescence spectrometry; g: inductively coupled plasma mass spectrometry; h: high-performance ion-exclusion chromatography; i: atomic absorption spectrometry
Analytical applications
The above described preconcentration/determination of Ge(IV) was applied to samples of seawater and water obtained after oil pumping, and a comparison with the results of the reference atom-absorption analysis indicates high accuracy and precision of the proposed methodology (Table 4).
Table 4. Determination of Ge (IV) in spiked different water samples.
| Sample | Ge(IV) added ng mL–1 | Proposed method | AAS method | t-testb | F-testc | ||
|---|---|---|---|---|---|---|---|
| Ge(IV) Founda | Recovery % | Ge(IV) Founda | Recovery % | ||||
| Sea Water d | 0 | 11.8 | --- | 12 | --- | ||
| 25 | 36.5 ± 0.81 | 99.18 | 37.6 ± 1.97 | 101.62 | 1.06 | 2.66 | |
| 50 | 61.2 ± 0.97 | 99.03 | 61.4 ±1.42 | 99.03 | 0.82 | 2.11 | |
| 60 | 72.4 ± 0.90 | 100.84 | 71.1 ± 1.48 | 98.75 | 0.72 | 1.98 | |
| Sea Water e | 0 | 12.5 | --- | 12.4 | --- | ||
| 20 | 32.8 ± 0.76 | 100.92 | 32.2 ± 1.84 | 99.38 | 1.87 | 3.76 | |
| 40 | 52.3 ± 0.92 | 99.62 | 52.9 ± 1.43 | 100.95 | 1.76 | 3.67 | |
| 60 | 72.1 ± 0.81 | 99.45 | 73.2 ± 1.29 | 101.11 | 1.95 | 3.83 | |
| Water obtained after oil pumpingf | 0 | 30.3 | --- | 30.5 | --- | ||
| 15 | 45.6 ± 0.87 | 100.66 | 44.9 ± 1.66 | 98.68 | 1.35 | 3.13 | |
| 30 | 60.8 ± 0.91 | 100.83 | 60.1 ± 1.48 | 99.34 | 1.66 | 3.59 | |
| 45 | 74.4 ± 1.38 | 98.81 | 76.2 ± 1.74 | 100.93 | 1.81 | 3.73 | |
Note: a Mean ± Relative Standard Deviation (n=6); b Tabulated t-value for five degrees of freedom at P (0.95) is 2.57; c Tabulated F-value at P (0.95) is 5.05; d Mediterranean sea from Alexandria; e Red sea from El-Ghardaka; f Water obtained after oil pumpingg
Germanium in the studied water samples exists at several ten ng L–1 levels [1,11], thus, upon the treatment, it was concentrated by 175 or 200 fold. The known amounts of Ge(IV) were also spiked to water samples before pretreatment. A good agreement between the determined and added amount of Ge(IV) has been obtained. Excellent recoveries were obtained, indicating the suitability of the sorbent for the selective collection of germanium from various water samples.
The performance of the proposed procedure was assessed by calculation of the t-value (for accuracy) and F-test (for precision) compared with the AAS method. The mean values were obtained in a Student’s t- and F-tests at 95% confidence limits for five degrees of freedom [41]. The results indicated that the calculated values (Table 4) did not exceed the theoretical values. The higher accuracy, wider range of determination, increased stability and lower time consumption indicate the advantages of the proposed method over the other procedure.
Conclusion
The results obtained demonstrate the efficiency of the chemically modified chitosan biopolymer sorbent towards selective quantitative sorption and preconcentration of Ge(IV). The sorption is markedly affected using complexing reagent, pH and surfactant, salt and time. Favorable features of the described methodology are its low instrument and running costs, simplicity, easy operation, sensitivity and high selectivity. In contrast to some other reported sorbents, the studied sorbent indicates better characteristics, as being superior in terms of selectivity, dynamic sorption capacity and detection limits, in addition to lower range of determination. The separation step results in an analytical sample which is relatively free of interferences of several ions. It can be successfully applied in routine analysis and can be applied for the determination of ultratrace amounts Ge (IV) in a diversity of objects (environmental samples) without significant interference from other cationic species present in the samples.
References
- Xiang L, Zhang X, Lu M, et al. Preconcentration of ultra-trace germanium in water samples with nano-sized TiO2 colloid and determination by GFAAS with colloid sampling. J Anal Atom Spect. 2012;27(2):359-63.
- Zhang L, Guo X, Li H, Yuan Z, et al. Separation of trace amounts of Ga and Ge in aqueous solution using nano-particles micro-column. Talanta. 2011;85(5):2463-9.
- Moskalyk RR. Review of germanium processing worldwide. Minerals Eng. 2004;17:(3)393-402.
- Kalderis D, Tsolaki E, Antoniou C, et al. Characterization and treatment of wastewater produced during the hydro-metallurgical extraction of germanium from fly ash. Desalination. 2008;230:162-174.
- Nukatsuka I, Takahashi K, Ohzeki K, et al. Solid-phase spectrophotometric determination of germanium on a membrane filter after collection using phenylfluorone and zephiramine. Analyst. 1989;114(11):1473-8.
- Harada A, Tarutani T, Yoshimura K. Spectrophotometric determination of germanium in rocks after selective adsorption on sephadex gel. Anal Chim Acta. 1988;209:333-8.
- Inukai Y, Kaida Y, Yasuda S. Selective separation of germanium(IV) by iminodiacetic acid-type chitosan chelating resin. Anal Sci. 1997;13:339-44.
- Göktüŕk G, Delzendeh M, Volkan M. Preconcentration of germanium on mercapto-modified silica gel. Spectrochim Acta B. 2005;65:1063-71.
- Inukai Y, Tanaka Y, Shiraishi Y, et al. Selective separation of germanium (IV) by di(2-hydroxyethyl)amine-type cellulose derivative. Anal Sci. 2001;17:1117–20 .
- Inukai Y, Chinen T, Matsuda T, et al. Selective separation of germanium(IV) by 2,3-dihydroxypropyl chitosan resin. Anal Chim Acta. 1998;371(2-3):187-93.
- Sabarudin A, Umemura T, Motomizu S. Chitosan functionalized with di-2-propanolamine: Its application as solid phase extractant for the determination of germanium in water samples by ICP-MS. Microchem J. 2011;99(1):34-39.
- Nashine N, Mishra RK. Selective extractive spectrophotometric determination of germanium with N-hydroxy-N,N’-diphenylbenzamidine and iodide. Anal Chim Acta. 1994;285:365-8.
- Nalini S, Ramakrishna TV. An indirect method for the spectrofluorimetric determination of trace amounts of germanium after extraction as an ion association complex with rhodamine B in the presence of chromotropic acid. Talanta. 1996;43:1437-41.
- Zhou X, Li ZJ, Yuan R, et al. A novel room temperature ionic liquid extraction spectrophotometric determination of trace germanium in natural water with methybenzeneazo-salicylfluorone. Anal Lett. 2006;39(5):863-77.
- Chen Y, Zhu R, Qiong H, et al. Determination of trace germanium by spectrophotometry after preconcentration on an organic solvent-soluble membrane. Michrochem J. 2000;64(1):93-7.
- Jianbo S, Zhiyong T, Chunhua T, et al. Determination of trace amounts of germanium by flow injection hydride generation atomic fluorescence spectrometry with on-line coprecipitation. Talanta. 2002;56(4):711-6.
- Brindle ID, Brindle ME, Le XC. Preconcentration by coprecipitation. Part 1. Rapid method for the determination of ultra-trace amounts of germanium in natural waters by hydride generation-atomic emission spectrometry. J Anal Atom Spect. 1991;6:129-32.
- Böyükbayram AE, Volkan M. Cloud point preconcentration of germanium and determination by hydride generation atomic absorption. Spectrochim Acta B. 2000;55:1073-80.
- Gao H-W, Liu W-G. Spectrophotometric investigation of germanium complex solution with o-chlorophenylfluorone and determination of trace amounts of germanium. Bull Korean Chem Soc. 2000;21(11):1090-4.
- Shen H, Wang Z, Xu G. Spectrophotometric determination of trace amounts of germanium in minerals and ores with 9-(o-chlorophenyl)-2,6,7-trihydroxyl-xanthen- 3-one in the presence of cetyltrimethylammonium bromide. Analyst. 1987;112(6):887-9.
- Soylak M, Yigit S. Preconcentration–separation of germanium at ultra-trace levels on polysulfone membrane filter and its determination by spectrophotometry. J Ind Eng Chem. 2015;24:322-5.
- Tomita H, Samukawa N, Asano M, et al. Spectrophotometric determination of germanium(IV) and organogermanes with o-sulfophenylfluorone. Bunseki kagaku. 2016;65(8):465-70.
- Zaijun L, Jiaomai P, Jan T. Spectrophotometric method for determination of germanium in foods with new color reagent trimethoxylphenylfluorone. Anal Chim Acta. 2001;445:153-9.
- Sun CQ, Gao Q, Liu LL. Adsorptive stripping measurements of germanium(IV) in the presence of pyrogallol. Talanta. 1995;42(7):881-4.
- Hernandis V, Macia L, Sala JV. Spectrophotometric determination of germanium and phosphorus in siliceous materials after hydrofluoric acid distillation. Analyst. 1987;112(7):1007-9.
- Matusiewicz H, Krawczyk-Coda M. Determination of germanium and tin and inorganic tin species by hydride generation in situ trapping flame atomic absorption spectrometry. Analytical Letters. 2010;43(16)2543-62.
- Zhang L, Li H, Liu X, et al. Sorption behavior of germanium(IV) on titanium dioxide nanoparticles. Russ J Inorg Chem. 2012;57(4):622-68.
- Zaijun L, Xia Z, Huizhen L, et al. Novel spectrophotometric method for determination of trace germanium in soils with methylbenzene-azosalicylfluorone using ultrasound-assisted leaching. Commun Soil Sci Plant Anal. 2008;39:461-74.
- Park HJ, Tavlarides LL. Germanium(IV) adsorption from aqueous solution using a Kelex-100 functional adsorbent. Ind Eng Chem Res. 2009;48(8):4014-21.
- Chen QC, Mou SF, Yan Y, et al. Separation and determination of inorganic germanium and β-carboxyethylgermanium sesquioxide by high-perform-ance ion-exclusion chromatography. J Chromatogr A. 1997;789:403-12.
- Torralvo FA, Fernández-Pereira C. Recovery of germanium from real fly ash leachates by ion-exchange extraction. Minerals Eng. 2011;24(1):35-41.
- Mahmudov KT, Aliyeva RA, Hamidov SZ, et al. Preconcentration of germanium (IV) on styrene-maleic anhydride copolymer modified with aminobenzoic acids and its spectrophotometric determination with bis(2,3,4-trihydroxyphenylazo) benzidine. Am J Anal Chem. 2012;3(12):790-9.
- Bayanov VA, Rakhimova OV, Rakhimov V, et al. Spectrophoto-metric differential kinetic method for the determination of germanium and silicon in the presence of each other in the GeO2–SiO2 systems. Glass Phy. & Chem. 2016;42(2):214-7.
- Marco-Lozar JP, Cazorla-Amoro D, Linares-Solano A. A new strategy for germanium adsorption on activated carbon by complex formation. Carbon. 2007;45(13):2519-28.
- Pokrovsky OS, Pokrovski GS, Schott J, et al. Experimental study of germanium adsorption on goethite and germanium coprecipitation with iron hydroxide: X-ray absorption fine structure and macroscopic characterization. Geochim Cosmochim Acta. 2006;70:3325-41.
- Gökmeşe F, Gökmeşe E, Solak AO. A new adsorptive square-wave stripping voltammetric method for the trace analysis of germanium. Hacettepe J Biol Chem. 2008;36(3):215-21.
- Broekaert JAC. Аnalytical atomic spectrometry with flames and plasmas. New York: Wiley. 2002.
- Britton HTS. Hydrogen ions. London: Chapman and Hall. 1952.
- Sparks DL. Environmental Soil Chemistry. New York: Academic Press. 1995.
- IUPAC. Nomenclature, symbols, units and their usage in spectrochemical analysis-II. data interpretation Analytical chemistry division. Spectrochim Acta B. 1978;33(6):241-5.
- Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. London: Prentice-Hall. 2005.