Review Paper - The International Tinnitus Journal (2017) Volume 21, Issue 2
Somatic Tinnitus
1Department of Oral and Maxillofacial Sciences, Sapienza University of Rome, Italy
2Department of Sense Organs, Sapienza University of Rome, Italy
3IRCCS Fondazione Ospedale San Camillo of Venice, Italy
Send correspondence to:
Massimo Ralli
Department of Oral and Maxillofacial Sciences, Sapienza University of Rome. Viale del Policlinico 155, 00186, Rome Italy, E-mail: massimo.ralli@uniroma1.it
Paper submitted to the ITJ-EM (Editorial Manager System) on September 26, 2017; and accepted on October 10, 2017.
Citation: Ralli M, Greco A, Cialente F, Di Stadio A, de Virgilio A, Longo L, Ciofalo A, Turchetta R, Cianfrone G, de Vincentiis M. Somatic Tinnitus. Int Tinnitus J. 2017;21(2):112-121
Abstract
Modulation of tinnitus characteristics such as pitch and loudness has been extensively described following movements of the head, neck and limbs, vertical or horizontal eye gaze, pressure on myofascial trigger points, cutaneous stimulation of the hands, electrical stimulation of the median nerve, and transcranial direct current stimulation. Modulation of tinnitus follows complex interactions between auditory and somatosensory afferents and can be favored by underlying somatic disorders. When tinnitus appears to be preceded or strictly linked to a somatic disorder, and therefore related to problems of the musculoskeletal system rather than of the ear, it is defined somatic tinnitus. A correct diagnosis and treatment of somatic disorders underlying tinnitus play a central role for a correct management of somatic tinnitus. However, the identification of somatic tinnitus may be complex in some cases. In this paper, after a general review of the current evidences for somatic tinnitus available in the literature, we present and discuss some cases of patients in which somatic modulation of tinnitus played a role–although different from case to case-in their tinnitus, describing the diagnostic and therapeutic approaches followed in each individual case and the results obtained, also highlighting unexpected findings and pitfalls that may be encountered when approaching somatic tinnitus patients.
Introduction
Tinnitus is defined as the perception of a sound in the absence of a matching external acoustic stimulus [1,2] and is considered a symptom rather than a disease [3].
Tinnitus is present in 11.9-30.3% of the adult population4,5, although only 0.5-3% refers to it as a condition that decreases quality of life [6,7]. Tinnitus prevalence increases with age up to 65-69 years, after which it decreases [8-14]. Social factors, such as lower income, poor education or occupational and recreational activity associated with noise exposure may influence the prevalence of tinnitus [15]. Tinnitus is regularly associated with hearing loss, which can be diagnosed in up to 90% of patients, and with the use of ototoxic drugs, infections, and medical conditions that can affect the hearing function triggering cochlear damage, with neural changes in the central auditory system [5,16-25]. These patients are considered to have otic tinnitus [2]; extensive research has been done to identify protective drugs and management strategies for patients with tinnitus and hearing loss [26-30].
Tinnitus can be evoked or modulated by inputs from the somato-sensory, somato-motor and visual– motor systems in some individuals [31-39]. This means that the psychoacoustic attributes of tinnitus (loudness and pitch) might change-though often only temporarilyfollowing external stimuli, such as the forceful muscle contractions of head, neck and limbs [31,40-44], orofacial movements [45], eye movements in the horizontal or vertical axis [46,47], pressure on myofascial trigger points [48,49], cutaneous stimulation of the hand/fingertip region [50], and of the face [43]; electrical stimulation of the median nerve and hand or finger movements [51]. Modulation of tinnitus represents a good example of central integration in the central nervous system, following interactions between auditory and somatosensory afferents occur as early in the auditory pathways as in the cochlear nucleus, at the site of convergence of the projections from the auditory nerve and trigeminal and dorsal column ganglia and brain stem nuclei.
Somatic modulation of tinnitus may be associated to underlying somatic disorders. When tinnitus appears to be preceded or strictly linked to a somatic disorder, and therefore related to problems of the musculoskeletal system rather than of the ear, it is defined somatic tinnitus [32,43,44,52].
Considerations on somatic modulation of tinnitus
Common risk factors for tinnitus are male gender, age and hearing problems [53-57]. Patients with somatic tinnitus have shown different characteristics, being younger, with higher prevalence of female gender and unrelated to hearing loss (somatic tinnitus patients often have normal hearing) or tinnitus severity [58-62]. The most common musculoskeletal conditions that underlie somatic tinnitus are temporomandibular joint (TMJ) and cervical spine (NECK) disorders [43,44,52].
Tinnitus modulation itself cannot be used as a single indicator for the somatic origin of tinnitus, hence identifying patients who could be treated with somatosensory systemrelated therapies. Levine [42] described this phenomenon as a “fundamental characteristic of tinnitus”, like its auditory and affective attributes. Somatic modulation has been reported in approximately two-thirds of tinnitus patients [35,43]; other studies revealed tinnitus modulation in 85%45, 83,3%42, 79%41, 57,9%32, 78%47, and 57% [61] of patients. A comparison of previous studies on tinnitus modulation is shown in Table 1.
| Author | Patients (#) | Year | Somatic Maneuvers (#) | Somatic Region | Prevalence of modulation (%) |
|---|---|---|---|---|---|
| Pinchoff et al [45] | 93 | 1998 | ns | TMJ, Head and Neck, Eye | 85 |
| Levine et al [31] | 70 | 1999 | 16 | TMJ, Head and Neck, Limb | 68 |
| Sanchez et al [33] | 121 | 2002 | 16 | TMJ, Head and Neck, Limb | 65.3 |
| Levine et al [42] | 62 | 2003 | 25 | TMJ, Head and Neck, Limb | 79 |
| Abel et al [41] | 60 | 2004 | 25 | TMJ, Head and Neck, Limb | 83.3 |
| Sanchez et al [32] | 38 | 2007 | 9 | Head and Neck | 57.9 |
| Simmons et al [47] | 45 | 2008 | 42 | TMJ, Head and Neck, Eye | 78 |
| An et al [64] | 45 | 2011 | 25 | TMJ, Head and Neck | 33.3 |
| Won et al [61] | 163 | 2013 | 19 | TMJ, Head and Neck | 57.1 |
| Ralli et al [65] | 310 | 2017 | 19 | TMJ, Head and Neck | 79.7 |
Average prevalence of modulation is 69%. Main somatic regions resulting in tinnitus modulation are temporomandibular joint (TMJ) and head and neck, followed by eye movements and limb. From Ralli et al, Somatosensory tinnitus: Current evidence and future perspectives [52].
Table 1: Comparison of previous studies on tinnitus modulation.
TMJ is the most common affected region in patients with somatic tinnitus. Rubinstein studied 102 individuals with tinnitus reporting that about one-third of the patients had influence on tinnitus by mandibular movements and/ or pressure applied to the temporomandibular joint63-65 and found that subjects with tinnitus had a significantly higher prevalence of cranio-mandibular disorders. Chole58 found tinnitus to be significantly more prevalent among a group of 338 patients with TMJ disorders compared to 694 controls. Kempf66 examined the TMJ and gnathological system of 138 patients with an inner ear disease, reporting that 13.8% of them had tinnitus and 79.7% had pathological findings: 44% had TMJ disorders, 29% parafunction of the occlusion and 35% a myopathy of the masticatory system.
The cervical spine and shoulder girdle are the second most frequent tinnitus-modulating region. Kapoula [67] reported that 61% of the patients examined in their clinic could modulate their tinnitus with jaw movements, 43% with head movements, 39% with muscle pressure, 13% with eye movements, and 9% with a global muscular effort. Application of head and neck maneuvers revealed that 41% of patients could only increase their tinnitus loudness, 17% could only decrease their tinnitus loudness, and 10% could either increase or decrease their tinnitus loudness depending upon the maneuver. In a recent study from our group, maneuvers on craniocervical region induced tinnitus loudness increase in 59,1% and decrease in 40,9% [65].
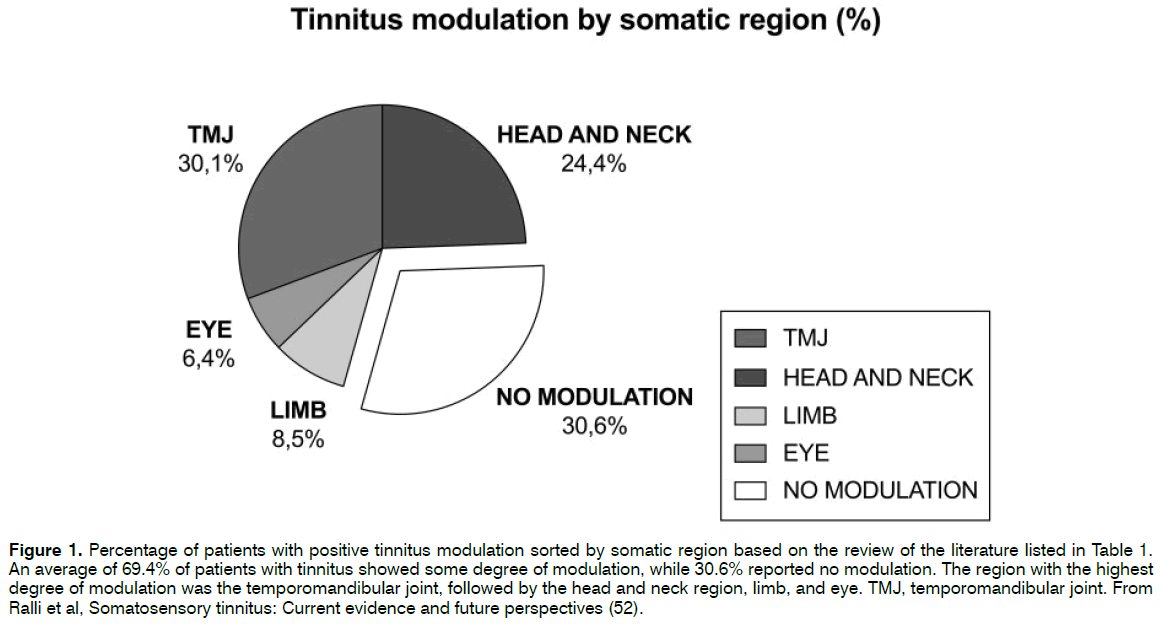
A percentage of positive tinnitus modulation sorted by somatic region based on a recently published literature review68 is shown in Figure 1.
Figure 1: Percentage of patients with positive tinnitus modulation sorted by somatic region based on the review of the literature listed in Table 1. An average of 69.4% of patients with tinnitus showed some degree of modulation, while 30.6% reported no modulation. The region with the highest degree of modulation was the temporomandibular joint, followed by the head and neck region, limb, and eye. TMJ, temporomandibular joint. From Ralli et al, Somatosensory tinnitus: Current evidence and future perspectives (52).
The identification of individuals that have an underlying somatic disorder contributing to their tinnitus onset and persistence is important when approaching tinnitus patients. Furthermore, once selected, a correct diagnosis and treatment of the somatic disorders underlying tinnitus is mandatory However, the identification of somatic tinnitus may be complex in some cases. In a previous paper from our group on 310 patients with somatic tinnitus [65], we found a significant association between positive history and positive tinnitus modulation for the same region, thus hypothesizing that such connection could help identify, among tinnitus patients, those with underlying head and neck dysfunctions that could play a role in their tinnitus, and who could benefit from further multidisciplinary investigation and physical therapy. In these cases, it is important to seek for cooperation of other specialists, such as dentists, gnathologists, osteopaths, orthopedics, physiotherapists for a second-level evaluation of a possible disorder affecting non-auditory regions.
Personal Experience
We report and comment on five exemplificative cases of patients presenting to the Tinnitus Unit of the Sapienza University in Rome, Italy, in which somatic modulation of tinnitus played a role in the diagnosis and treatment of their tinnitus. In all cases, we evaluated audiological history, tinnitus characteristics, self-administered questionnaire scores, somatic dysfunction history and tinnitus modulation following a set of maneuvers as previously published65. A detailed description of the maneuvers used for somatic modulation examination is listed in Table 2. When positive history and modulation was found, patients were referred to the Service of Clinical Gnathology of the Oral and Maxillofacial Surgery Unit of our University for clinical TMJ and NECK evaluation.
| Jaw Maneuvers | ||
|---|---|---|
| TMJ 1 | Clench teeth together | performed by patient |
| TMJ 2 | Open the mouth with restorative pressure | performed by examiner |
| TMJ 3 | Protrude jaw with restorative pressure | performed by examiner |
| TMJ 4 | Slide jaw to left with restorative pressure | performed by examiner |
| TMJ 5 | Slide jaw to right with restorative pressure | performed by examiner |
| Neck Maneuvers | ||
| NECK 1 | Resist pressure applied to the forehead | performed by examiner |
| NECK 2 | Resist pressure applied to the occiput | performed by examiner |
| NECK 3 | Resist pressure applied to the vertex | performed by examiner |
| NECK 4 | Resist pressure applied under the mandibule | performed by examiner |
| NECK 5 | Resist pressure applied to the right temple | performed by examiner |
| NECK 6 | Resist pressure applied to the left temple | performed by examiner |
| NECK 7 | Pressure to the right zygoma with head turned right | performed by examiner |
| NECK 8 | Pressure to the left zygoma with head turned left | performed by examiner |
| NECK 9 | Pressure to the left temple with head turned right and tilted to the left (left sternocleidomastoid muscle) | performed by examiner |
| NECK 10 | Pressure to the right temple with head turned left and tilted to the right (right sternocleidomastoid muscle) | performed by examiner |
| NECK 11 | Forward flection of the neck | performed by patient |
| NECK 12 | Backward flection of the neck | performed by patient |
| NECK 13 | Turn head to the right | performed by patient |
| NECK 14 | Turn head to the left | performed by patient |
Maneuvers used for somatic testing in our study as recently published in a previous work of the authors65. Some were performed by patient, some by the examiner (shown next to each maneuver). During somatosensory examination, patients were asked to perform a specific movement or to resist to a pressure applied by the examiner against the head, neck and jaw. Each contraction was held for 10 seconds; in case of positive tinnitus modulation examiner waited for tinnitus to return to baseline levels before proceeding with another maneuver. Maneuvers were performed in the same order for each patient.
Table 2: Comparison of previous studies on tinnitus modulation.
Case 1
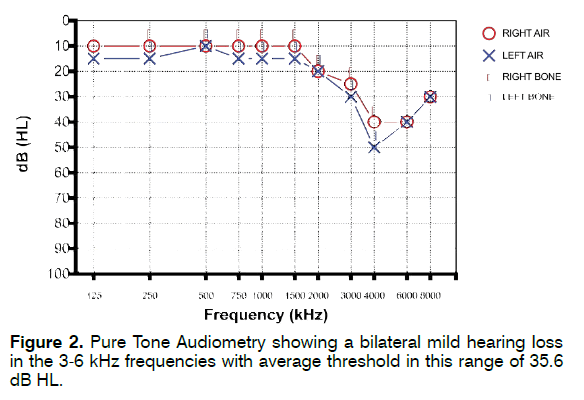
A 43-year-old man lamenting persistent bilateral tinnitus in the high-pitch from 8 years presented to our Tinnitus Unit. He reported chronic work-related noise exposure for several years in his twenties (manufacturing industry). Otoscopic examination was normal. His Pure Tone Audiometry (PTA) showed a bilateral mild hearing loss in the 3-6 kHz frequencies (more evident in the 4-6 kHz range) with average threshold in this range of 35.6 dB HL (Figure 2); the left ear showed slightly worse hearing compared to the right ear. Tinnitus was high pitch; tinnitogram measured using a pitch-match test showed a tinnitus pitch near 6 kHz. Tinnitus Handicap Inventory (THI) score was 28. The patient did not report history of TMJ or NECK disorders. When performing somatic tinnitus maneuvers, tinnitus loudness could be modulated for most TMJ (3/5-60%) and, to a lesser extent, NECK maneuvers (5/19-26.3%). The patient was referred to the Service of Clinical Gnathology of the Oral and Maxillofacial Surgery Unit of our University for TMJ and NECK evaluation; no clinically evident somatic disorders were found.
Comments on this case
This case is an example of a patient with auditory tinnitus most probably deriving from peripheral inner ear damage due to previous exposure to loud sounds. Tinnitus appeared about 10 years after prolonged noise exposure, as often seen in similar cases [15,69-73]. Although tinnitus could be successfully modulated with both TMJ and NECK maneuvers, no somatic disorder was found at a clinical level. Furthermore, this patient did not self-report history for somatic dysfunctions. This case demonstrates that tinnitus modulation can be found even when no somatic disorder is present; in fact, somatic modulation of tinnitus is a widespread condition that can be present with or without underlying somatic disorders [31,40]. Furthermore, as previously discussed, several authors reported a large capability of somatic tinnitus modulation in multiple patient series ranging between 65.3% and 83.3% [32,35,41-43,45,47,61,65]. In this patient, the negative history for self-reported somatic disorder suggests caution while taking into account a somatic origin for his tinnitus.
Case 2
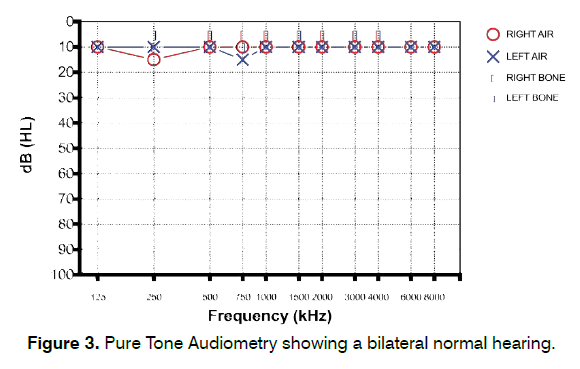
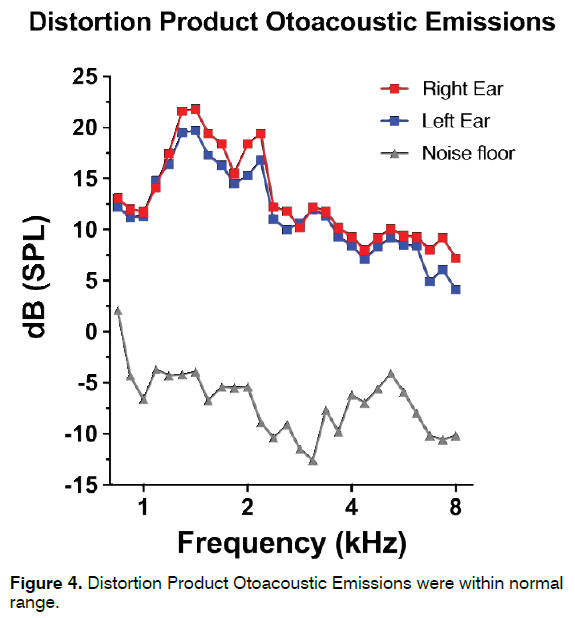
A 22-year-old woman reporting continuous, lowpitch, left-sided tinnitus from two years was admitted to our center. No significant noise exposure was described by the patient. Otoscopy and PTA were normal (Figure 3). Distortion Product Otoacoustic Emissions (DPOAE) were recorded in both ears and appeared within normal range (Figure 4). Tinnitogram showed low-pitched tinnitus with a frequency between 250 and 500 Hz. The patient reported a 3-year history of bruxism during night and TMJ pain in the morning; symptoms started between the last year of high school and the beginning of her university studies. THI score was 52, further psychological evaluation revealed an anxious phenotype. Somatic modulation was positive mainly for TMJ, with increased tinnitus loudness in 4/5 (80%) TMJ maneuvers and in 2/14 (14.3%) NECK maneuvers. Gnathological examination revealed the presence of a clinically evident TMJ disorder following DC/TMD Axis I classification [74-78] (Myalgia, Myofascial pain - ICD-9 729.1; Arthralgia - ICD-9 524.62). The patient was treated with a nocturnal occlusal splint for a period of 12 months, reporting a significant improvement in bruxism and TMJ pain and a complete resolution of her tinnitus about 8 months after initial assessment. THI score recorded 12 months after first admission to our clinic was 14.
Comments on this case
This is a typical case of somatic tinnitus following a TMJ disorder. Bruxism is also strongly linked to the stress and anxiety disorder of the patient that coincided with a critical time in her life (end of high-school studies with final exams, and beginning of a new cycle of education) [75-78]. At first examination, there were many factors suggesting the presence of somatic tinnitus. Normal hearing, normal DPOAE and no history of noise exposure almost ruled out the presence of auditory tinnitus although high-frequency (> 8 kHz) hearing loss was not studied; when evaluating somatic history and modulation of tinnitus, a clear match was found between self-reported history for TMJ dysfunction and tinnitus modulation in the TMJ region. Furthermore, female sex and unilateral tinnitus have been described to be more associated to somatic tinnitus [61]. The approach with this patient has been centered on treating the gnathological condition, with the use of an occlusal splint. Results on TMJ dysfunction treatment and, especially, on tinnitus have been very good, although tinnitus disappearance occurred after a rather long time (6 months) from the beginning of gnanthological treatment. It is therefore important, for a better compliance, to discuss with patients that begin a somatic treatment for their tinnitus that timing plays a central role in the effects on tinnitus perception, and somatic treatment should not be discontinued if tinnitus perception does not change in the short or medium term.
Case 3
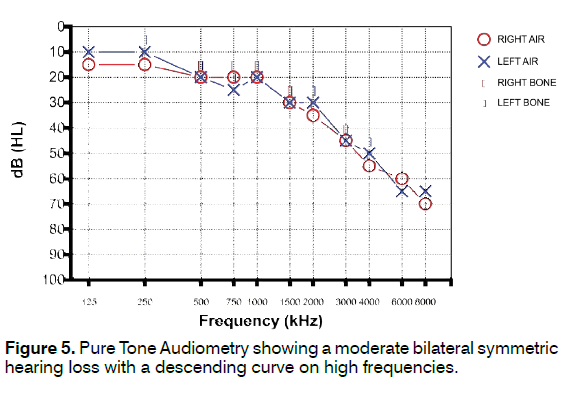
A 74-year-old woman presented to our tinnitus unit with a long history of bilateral high-pitched tinnitus more annoying in the left ear. Otoscopy was normal for age. PTA showed a moderate bilateral symmetric hearing loss with a descending curve on high frequencies (Figure 5). Speech discrimination was consistent with PTA. Tinnitogram showed a high pitch tinnitus around 3 kHz. THI score was 38, Hearing Handicap Inventory (HHI) score was 50. Self-reported somatic history was positive for TMJ and NECK dysfunctions; patient reported a bilateral TMJ click occurring from at least 10 years associated to TMJ pain when chewing, as well as chronic cervical pain more evident in the morning and upon awakening from a nap, probably due to somatic factors like stretching of the neck muscles when her head passively falls forward while sleeping in a sitting position. Tinnitus modulation was strongly positive resulting in an increased loudness following 5/5 (100%) TMJ maneuvers and decreased loudness following 12/14 (85.7%) NECK maneuvers. Patient was referred to multidisciplinary somatic evaluation to the Gnathology Service of our University; diagnosis of a clinically evident TMJ disorder was made (Disc displacement with reduction with intermittent locking - ICD-9 524.63; Degenerative joint disease - ICD-9 715.18; Myalgia, Myofascial pain - ICD-9 729.1); associated to C4-C5 herniation seen with cervical Magnetic Resonance Imaging. Patient was treated with occlusal splint and physical cervical treatment with heat application, deep tissue massage, electrical stimulation, and ultrasound in the cranio-cervical region for 6 months with significant improvement in her somatic symptoms. In addition, antioxidant drugs were administered at cycles for a period of 6 months. At the 6-month tinnitus evaluation in our center, the patient reported lower tinnitus annoyance (THI = 22) and slightly reduced self-perceived tinnitus loudness. No significant changes were found in hearing threshold.
Comments on this case
In this case, a combination of auditory and somatic tinnitus can be found: the somatic component plays a role in tinnitus and sums to the probable effects of presbycusis resulting in increased loudness and annoyance of her tinnitus. The identification of a somatic origin for her tinnitus thanks to the matching of self-reported history and modulation in the same somatic regions helped in addressing this patient to multidisciplinary somatic evaluation and treatment. Furthermore, the characteristics of tinnitus modulation found in this patient are consistent with what reported in the literature by some authors [32,33,41,42]. TMJ maneuvers induced an increase in tinnitus loudness, while NECK maneuvers induced a decrease of loudness. In a previous study from our group65 we also found that maneuvers on TMJ mainly resulted in increased loudness of tinnitus (94.3%), while maneuvers on the cranio-cervical region induced tinnitus loudness increase in 59.1% and decrease in 40.9%. Due to the multiple causes of tinnitus in this patient, the persistence of tinnitus found 6 months after initial assessment should be expected; however, a correct identification and treatment of the somatic components probably contributed in the reduction of tinnitus loudness and annoyance and improved quality of life of this patient.
Case 4
An 18-year-old man with persistent single-sided “buzzing” tinnitus in the right ear started 2 years earlier presented to our center. No exposure to loud sounds was disclosed. The patient also reported reduced tolerance to sounds of moderate intensity in day-by-day activities that induced him to avoid social events and significantly limited his daily activities.
Tinnitus started right after a maxillofacial trauma with severe psychological correlations: in fact, he was a victim of street violence being beaten for unknown reasons. He was hospitalized for 18 days and diagnosed with fracture of the right zygomatic bone that required surgical intervention. After trauma, the patient was diagnosed with post-traumatic stress disorder and was assisted by a psychologist for two years. Hyperacusis symptoms started about 6 months after the onset of tinnitus.
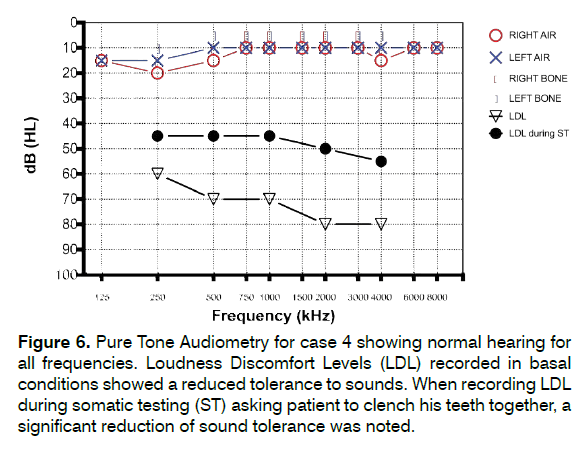
PTA and DPOAE were within normal range. THI score was very high (score = 86), Hyperacusis Questionnaire (HQ) score was 34 and Gerauschuberempfindlichkeit Questionnaire (GUF) score was 41. Loudness Discomfort Levels (LDL) recorded in basal conditions showed a reduced tolerance to sounds. We also performed LDL while asking patient to clench his teeth together: interestingly, a remarkable further reduction of sound tolerance was noted (Figure 6). Tinnitus modulation was positive for 5/5 (100%) TMJ maneuvers and 18/19 (94.7%) NECK maneuvers.
Figure 6: Pure Tone Audiometry for case 4 showing normal hearing for all frequencies. Loudness Discomfort Levels (LDL) recorded in basal conditions showed a reduced tolerance to sounds. When recording LDL during somatic testing (ST) asking patient to clench his teeth together, a significant reduction of sound tolerance was noted.
During interview, the patient defined his situation as follows: “Sounds penetrate every aspect of my life, and their presence causes pain; the duration of exposure contributes to the intensity of the pain. The more loud and long sounds are, the more pain is acute and longlasting. Even the lowest and most imperceptible sounds are amplified and distorted in such a way to invade every little aspect of my daily activities. Sometimes, I cannot even talk. I noticed a sharp decrease in the threshold of pain caused by sound”. Furthermore, the patient carefully described a list of daily activities, for both his private and social life, that were subjectively strongly limited by the hyperacusis condition. The complete list is shown in Table 3.
| Private Life | |
|---|---|
| Listen to music | |
| Watch movies | |
| Get out of the house if it is raining | |
| Stay in the bathroom while flushing | |
| Stay in the kitchen if there are fries that make noise while cooking | |
| Be in a room with noises of plates and glasses slipped involuntarily | |
| Be in a room with noises of electronic tools such as washing machine, blender, electric razor, aerosol machine and vacuum cleaners | |
| Dog barking or bird chirping | |
| Play musical instruments | |
| Sing or just raise the voice | |
| Use the whistle | |
| Social Life and Studying | |
| Use headphones | |
| Attend a concert of any kind | |
| Attend sporting events | |
| Go to cinema | |
| Go to disco | |
| Stay in a pub, restaurant or bar with friends with music background | |
| Stay on busy roads (e.g. shopping streets or crowded squares) | |
| Stay close to truck engines or ambulances | |
| Stay in close proximity to airports | |
| Stay near the dock on the arrival of the subway train | |
| Climb on wagons of loud public transport (e.g. glasses and doors that shake and bump on buses, non-sounded engines, metro wagons with open windows) | |
| Go to parties, social gatherings, festivals or other noisy events | |
| Talk to other people for several consecutive minutes | |
| Repeat aloud while studying | |
| Attend university lectures in large classrooms where microphone is needed | |
| Attend a demonstration | |
| Participate in public competitions where a microphone is needed | |
| Participate in book or movie presentations | |
Table 3: List of daily activities limited by hyperacusis in case 4.
Patient was addressed to second level gnathological evaluation that found no residual consequences of the maxillo-facial trauma, completely resolved without consequences on TMJ and NECK; no other somatic disorders requiring treatment were found. Based on these results, the patient was addressed to psychiatric evaluation and cognitive behavioral therapy was proposed as a treatment.
Comments on this case
This represents a complicated case of a young patient in which somatic and psychological factors contributed to development of a highly annoying tinnitus associated to hyperacusis that significantly affected his daily activities. In this case, although history was strongly suggestive of a somatic origin of tinnitus, the psychological element assumed over time a higher and, when approaching our tinnitus unit, prevalent role. This is even more evident while reading the list of daily activities described by the patient as strongly limited by the hyperacusis (Table 3).
Somatic modulation was impressively high in this case, and apparently had a role in further reducing sound tolerance as shown by LDL threshold performed during teeth clenching. However, no residual somatic disorder was found at ghathological examination.
Schecklmann [79] evaluated the prevalence of somatic modulation in patients with and without hyperacusis, finding it significantly higher in hyperacusic patients. The authors also reported a significantly higher presence of self-reported somatic history in hyperacusis patients. The increased prevalence of somatic modulation found by the authors in hyperacusis patients could be due to increased peripheral somatic activation or central hypersensitivity to somatic inputs. The latter is supported by neurophysiological findings that show increased sensitivity to multisensory stimuli in patients with hyperacusis, which may be linked to a hypervigilance network [80-84]. Also, Schecklmann [79] and Gilles85 found worse tinnitus and depression scores in patients with hyperacusis than in those without. Higher tinnitus loudness, discomfort and annoyance could be therefore explained by the involvement of emotion-related neural circuits [86-90]. This evidence suggests that, when evaluating somatic tinnitus patients, clinicians should consider the possible amplification of the somatic component by comorbid hyperacusis and other associated conditions, as hyperacusis could result from a generalized hypersensitivity disorder involving multiple sensory pathways. Therefore, it is recommended to determine if hyperacusis is present in patients with somatic tinnitus, to carefully select patients whose tinnitus would benefit from a somatic therapy.
Case 5
52-year-old woman with a 6-month history of right sided low-pitched tinnitus presented to our Tinnitus Center. PTA showed a mild bilateral hearing loss in the high frequencies (4-8 kHz) with average threshold in this range of 28.3 dB HL (Figure 7). Otoscopic examination was normal. Tinnitogram showed a tinnitus pitch around 1 kHz. THI score was 16, HHI was 14. She reported a longtime history of bilateral TMJ clicking with three episodes of subluxation of the mandible requiring medical assistance. Somatic tinnitus maneuvers were slightly positive for TMJ (increased loudness in 1/5-20%) and strongly positive for NECK (decreased loudness in 14/19-73.7%). The patient was referred to a gnathologist for somatic evaluation; she received a diagnosis of TMJ disorder (Degenerative joint disease - ICD-9 715.18; Subluxation - ICD-9 830.1; Disc displacement with reduction with intermittent locking - ICD-9 524.63), while no NECK disorders were found. The patient was treated with dental splint and myorelaxant drugs for 6 months. When tinnitus was evaluated 6 months after the beginning of somatic treatment, the patient reported a significantly lower loudness and annoyance of tinnitus, however still present, with a THI score of 12 and HHI of 14. A new somatic tinnitus modulation examination revealed a reduction of NECK positive maneuvers (3/19- 15.8%).
Comments on this case
This is a case of somatic tinnitus in which somatic modulation did not match self-reported history of somatic dysfunction, and may confuse the examiner. In fact, although modulation was strongly present in the NECK region, no NECK disorder was found; instead, a severe TMJ disorder was diagnosed even if TMJ modulation was mild (only 1 positive maneuver out of 5). However, after treating the TMJ disorder, a reduction in modulation following somatic maneuvers in the NECK region was found. This could be explained by the possible effect of the TMJ disorder on the NECK ascending pathways, resulting in a modulation in this region as well [91-94]. This suggests to carefully evaluate the somatic component, especially when a nos history of TMJ dysfunction is suspected and no other significant risk factors are present.
Conclusion
Current literature and clinical experience confirm the wide presence of somatic modulation of tinnitus, thus rising interest on when this should be considered as an indicator of an underlying somatic disorder that requires multidisciplinary diagnostic and therapeutic approach. The cases presented in this paper, although representing only a small part of the case histories observed in our center, are shown as examples of the many variables that can be encountered in daily clinical practice with tinnitus patients, and suggest caution in relying on tinnitus modulation alone to define patient treatment. When a somatic disorder is suspected, however, a multidisciplinary approach is encouraged, as somatic disorders have been shown to play a role in a large portion of tinnitus sufferers and, when correctly identified and treated, represent a valid therapeutic option for tinnitus treatment.
Acknowledgments
We thank Italian Association for Research on Deafness (AIRS Onlus) for support in the management of patients.
Conflicts of Interest and Source of Funding
The authors declare that they have no conflicts of interest. The authors have not received financial support for this research.
References
- Haider HF, Hoare DJ, Costa RFP, Potgieter I, Kikidis D, Lapira A, et al. Pathophysiology, diagnosis and treatment of somatosensory tinnitus: A scoping review. Front Neurosci. 2017;11:207.
- Roberts LE, Eggermont JJ, Caspary DM, Shore SE, Melcher JR, Kaltenbach JA. Ringing ears: The neuroscience of tinnitus. J Neurosci. 2010;30(45):14972-9.
- Jastreboff PJ, Hazell JW. A neurophysiological approach to tinnitus: Clinical implications. Br J Audiol. 1993;27(1):7-17.
- McCormack A, Edmondson-Jones M, Somerset S, Hall D. A systematic review of the reporting of tinnitus prevalence and severity. Hear Res. 2016;337:70-9.
- Moller AR. Epidemiology of tinnitus in adults. Textbook of Tinnitus. 2011:29-37.
- Coles RR. Epidemiology of tinnitus: (1) prevalence. J Laryngol Otol Suppl. 1984;9:7-15.
- Swain SK, Nayak S, Ravan JR, Sahu MC. Tinnitus and its current treatment-Still an enigma in medicine. J Formos Med Assoc. 2016;115(3):139-44.
- Nondahl DM, Cruickshanks KJ, Wiley TL, Klein R, Klein BE, Tweed TS. Prevalence and 5-year incidence of tinnitus among older adults: the epidemiology of hearing loss study. J Am Acad Audiol. 2002;13(6):323-31.
- Sindhusake D, Mitchell P, Newall P, Golding M, Rochtchina E, Rubin G. Prevalence and characteristics of tinnitus in older adults: The blue mountains hearing study. Int J Audiol. 2003;42(5):289-94.
- Hoffman HJ, Dobie RA, Losonczy KG, Themann CL, Flamme GA. Declining prevalence of hearing loss in us adults aged 20 to 69 years. JAMA Otolaryngol Head Neck Surg. 2017;143(3):274-85.
- Al-Swiahb J, Park SN. Characterization of tinnitus in different age groups: A retrospective review. Noise Health. 2016;18(83):214-9.
- Ferreira LM, Ramos Junior AN, Mendes EP. Characterization of tinnitus in the elderly and its possible related disorders. Braz J Otorhinolaryngol. 2009;75(2):249-55.
- Rosenhall U, Karlsson AK. Tinnitus in old age. Scand Audiol. 1991;20(3):165-71.
- Stouffer JL, Tyler RS. Characterization of tinnitus by tinnitus patients. J Speech Hear Disord. 1990;55(3):439-53.
- Ralli M, Balla MP, Greco A, Altissimi G, Ricci P, Turchetta R, et al. Work-related noise exposure in a cohort of patients with chronic tinnitus: Analysis of demographic and audiological characteristics. Int J Environ Res Public Health. 2017;14(9).
- Langguth B, Kreuzer PM, Kleinjung T, De Ridder D. Tinnitus: Causes and clinical management. Lancet Neurol. 2013;12(9):920-30.
- Crummer RW, Hassan GA. Diagnostic approach to tinnitus. Am Fam Physician. 2004;69(1):120-6.
- Sheppard A, Hayes SH, Chen GD, Ralli M, Salvi R. Review of salicylate-induced hearing loss, neurotoxicity, tinnitus and neuropathophysiology. Acta Otorhinolaryngol Ital. 2014;34(2):79-93.
- Chen GD, Kermany MH, D'Elia A, Ralli M, Tanaka C, Bielefeld EC, et al. Too much of a good thing: Long-term treatment with salicylate strengthens outer hair cell function but impairs auditory neural activity. Hear Res. 2010;265(1-2):63-9.
- Ralli M, Troiani D, Podda MV, Paciello F, Eramo SL, De Corso E, et al. The effect of the NMDA channel blocker memantine on salicylate-induced tinnitus in rats. Acta Otorhinolaryngol Ital. 2014;34(3):198-204.
- Fetoni AR, Ralli M, Sergi B, Parrilla C, Troiani D, Paludetti G. Protective properties of antioxidant drugs in noise-induced hearing loss in the guinea pig. Audiological Medicine. 2009;6:271-7.
- Di Stadio A, Ralli M. Systemic Lupus Erythematosus and hearing disorders: Literature review and meta-analysis of clinical and temporal bone findings. J Int Med Res. 2017:300060516688600.
- Ralli M, Greco A, Falasca V, Tombolini M, Turchetta R, De Fazio S, et al. Recovery from repeated sudden hearing loss in a patient with Takayasu’s Arteritis treated with hyperbaric oxygen therapy: The first report in the literature. Case Rep Otolaryngol. 2017.
- Ralli M, Altissimi G, Di Stadio A, Mazzei F, Turchetta R, Cianfrone G. Relationship between hearing function and myasthenia gravis: A contemporary review. J Int Med Res. 2016.
- Ralli M, Di Stadio A, Greco A, Altissimi G, Mazzei F, Turchetta R, et al. Development of progressive hearing loss and tinnitus in a patient with myasthenia gravis: an overlooked comorbidity? Hearing, Balance and Communication. 2017;1-7.
- Ralli M, Lobarinas E, Fetoni AR, Stolzberg D, Paludetti G, Salvi R. Comparison of salicylate- and quinine-induced tinnitus in rats: Development, time course, and evaluation of audiologic correlates. Otol Neurotol. 2010;31(5):823-31.
- Fetoni AR, Mancuso C, Eramo SL, Ralli M, Piacentini R, Barone E, et al. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience. 2010;169(4):1575-88.
- Fetoni AR, Ralli M, Sergi B, Parrilla C, Troiani D, Paludetti G. Protective effects of N-acetylcysteine on noise-induced hearing loss in guinea pigs. Acta Otorhinolaryngol Ital. 2009;29(2):70-5.
- Fetoni AR, Garzaro M, Ralli M, Landolfo V, Sensini M, Pecorari G, et al. The monitoring role of otoacoustic emissions and oxidative stress markers in the protective effects of antioxidant administration in noise-exposed subjects: A pilot study. Med Sci Monit. 2009;15(11):PR1-8.
- Le TN, Straatman LV, Lea J, Westerberg B. Current insights in noise-induced hearing loss: A literature review of the underlying mechanism, pathophysiology, asymmetry, and management options. J Otolaryngol Head Neck Surg. 2017;46(1):41.
- Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 1999;20(6):351-62.
- Sanchez TG, Da Silva Lima A, Brandao AL, Lorenzi MC, Bento RF. Somatic modulation of tinnitus: Test reliability and results after repetitive muscle contraction training. Ann Otol Rhinol Laryngol. 2007;116(1):30-5.
- Sanchez TG, Guerra GC, Lorenzi MC, Brandao AL, Bento RF. The influence of voluntary muscle contractions upon the onset and modulation of tinnitus. Audiol Neurootol. 2002;7(6):370-5.
- Coad ML, Lockwood A, Salvi R, Burkard R. Characteristics of patients with gaze-evoked tinnitus. Otol Neurotol. 2001;22(5):650-4.
- Shore SE, Roberts LE, Langguth B. Maladaptive plasticity in tinnitus--triggers, mechanisms and treatment. Nat Rev Neurol. 2016;12(3):150-60.
- Wu C, Stefanescu RA, Martel DT, Shore SE. Tinnitus: Maladaptive auditory-somatosensory plasticity. Hear Res. 2016;334:20-9.
- Shore SE. Plasticity of somatosensory inputs to the cochlear nucleus-implications for tinnitus. Hear Res. 2011;281(1-2):38-46.
- Dehmel S, Cui YL, Shore SE. Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am J Audiol. 2008;17(2):S193-209.
- Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog Brain Res. 2007;166:107-23.
- Levine RA, Nam EC, Oron Y, Melcher JR. Evidence for a tinnitus subgroup responsive to somatosensory based treatment modalities. Prog Brain Res. 2007;166:195-207.
- Abel MD, Levine RA. Muscle contractions and auditory perception in tinnitus patients and nonclinical subjects. Cranio. 2004;22(3):181-91.
- Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153(4):643-8.
- Sanchez TG, Rocha CB. Diagnosis and management of somatosensory tinnitus: review article. Clinics (Sao Paulo). 2011;66(6):1089-94.
- Ralli MA, G.; Turchetta, R.; Cianfrone, G. Somatic modulation of tinnitus: A review and some open questions. Otolaryngol Open J. 2016;2(4):111-4.
- Pinchoff RJ, Burkard RF, Salvi RJ, Coad ML, Lockwood AH. Modulation of tinnitus by voluntary jaw movements. Am J Otol. 1998;19(6):785-9.
- Baguley DM, Phillips J, Humphriss RL, Jones S, Axon PR, Moffat DA. The prevalence and onset of gaze modulation of tinnitus and increased sensitivity to noise after translabyrinthine vestibular schwannoma excision. Otol Neurotol. 2006;27(2):220-4.
- Simmons R, Dambra C, Lobarinas E, Stocking C, Salvi R. Head, neck, and eye movements that modulate tinnitus. Semin Hear. 2008;29(4):361-70.
- Rocha CB, Sanchez TG. Efficacy of myofascial trigger point deactivation for tinnitus control. Braz J Otorhinolaryngol. 2012;78(6):21-6.
- Rocha CA, Sanchez TG. Myofascial trigger points: Another way of modulating tinnitus. Prog Brain Res. 2007;166:209-14.
- Cacace AT. Expanding the biological basis of tinnitus: Crossmodal origins and the role of neuroplasticity. Hear Res. 2003;175(1-2):112-32.
- Cullington H. Tinnitus evoked by finger movement: brain plasticity after peripheral deafferentation. Neurology. 2001;56(7):978.
- Ralli M, Greco A, Turchetta R, Altissimi G, De Vincentiis M, Cianfrone G. Somatosensory tinnitus: Current evidence and future perspectives. J Int Med Res. 2017:300060517707673.
- Oostendorp RA, Bakker I, Elvers H, Mikolajewska E, Michiels S, De Hertogh W, et al. Cervicogenic somatosensory tinnitus: An indication for manual therapy plus education? Part 2: A pilot study. Man Ther. 2016;23:106-13.
- Eggermont JJ, Roberts LE. The neuroscience of tinnitus. Trends Neurosci. 2004;27(11):676-82.
- Baguley D, McFerran D, Hall D. Tinnitus. Lancet. 2013;382(9904):1600-7.
- Gopinath B, McMahon CM, Rochtchina E, Karpa MJ, Mitchell P. Incidence, persistence, and progression of tinnitus symptoms in older adults: The blue mountains hearing study. Ear Hear. 2010;31(3):407-12.
- Shargorodsky J, Curhan GC, Farwell WR. Prevalence and characteristics of tinnitus among US adults. Am J Med. 2010;123(8):711-8.
- Chole RA, Parker WS. Tinnitus and vertigo in patients with temporomandibular disorder. Arch Otolaryngol Head Neck Surg. 1992;118(8):817-21.
- Wright EF, Bifano SL. The relationship between tinnitus and temporomandibular disorder (TMD) therapy. Int Tinnitus J. 1997;3(1):55-61.
- Vielsmeier V, Kleinjung T, Strutz J, Burgers R, Kreuzer PM, Langguth B. Tinnitus with temporomandibular joint disorders: A specific entity of tinnitus patients? Otolaryngol Head Neck Surg. 2011;145(5):748-52.
- Won JY, Yoo S, Lee SK, Choi HK, Yakunina N, Le Q, et al. Prevalence and factors associated with neck and jaw muscle modulation of tinnitus. Audiol Neurootol. 2013;18(4):261-73.
- Ward J, Vella C, Hoare DJ, Hall DA. Subtyping somatic tinnitus: A Cross-sectional UK cohort study of demographic, clinical and audiological characteristics. PLoS One. 2015;10(5):e0126254.
- Rubinstein B, Axelsson A, Carlsson GE. Prevalence of signs and symptoms of craniomandibular disorders in tinnitus patients. J Craniomandib Disord. 1990;4(3):186-92.
- An YH CA, Yoon S, Shim H. Comparison of clinical characteristics and somatic modulation between somatic tinnitus and otic tinnitus. Audiol Neurootol Extra. 2011;1:9-19.
- Ralli M, Altissimi G, Turchetta R, Mazzei F, Salviati M, Cianfrone F, et al. Somatosensory tinnitus: Correlation between cranio-cervico-mandibular disorder history and somatic modulation. Audiol Neurootol. 2016;21(6):372-82.
- Kempf HG RR, Mühlbradt L. Correlation between inner ear disorders and temporomandibular joint diseases. HNO. 1993;41(1):7-10.
- Kapoula Z, Yang Q, Le TT, Vernet M, Berbey N, Orssaud C, et al. Medio-lateral postural instability in subjects with tinnitus. Front Neurol. 2011;2:35.
- Ralli M, Greco A, Turchetta R, Altissimi G, De Vincentiis M, Cianfrone G. Somatosensory tinnitus: Current evidence and future perspectives. J Int Med Res. 2017;45(3):933-47.
- Sha SH, Schacht J. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin Investig Drugs. 2017;26(1):85-96.
- Frederiksen TW, Ramlau-Hansen CH, Stokholm ZA, Grynderup MB, Hansen AM, Lund SP, et al. Occupational noise exposure, psychosocial working conditions and the risk of tinnitus. Int Arch Occup Environ Health. 2017;90(2):217-25.
- Wells TS, Seelig AD, Ryan MA, Jones JM, Hooper TI, Jacobson IG, et al. Hearing loss associated with US military combat deployment. Noise Health. 2015;17(74):34-42.
- Lobarinas E, Salvi R, Baizer J, Altman C, Allman B. Noise and health special issue: Advances in the neuroscience of tinnitus. Noise Health. 2013;15(63):81-2.
- Tak S, Davis RR, Calvert GM. Exposure to hazardous workplace noise and use of hearing protection devices among US workers--NHANES, 1999-2004. Am J Ind Med. 2009;52(5):358-71.
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, Goulet JP, et al. Diagnostic criteria for temporomandibular disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD consortium network* and orofacial pain special interest groupdagger. J Oral Facial Pain Headache. 2014;28(1):6-27.
- Cavallo P, Carpinelli L, Savarese G. Perceived stress and bruxism in university students. BMC Res Notes. 2016;9(1):514.
- Klasser GD, Rei N, Lavigne GJ. Sleep bruxism etiology: The evolution of a changing paradigm. J Can Dent Assoc. 2015;81:f2.
- Wieckiewicz M, Paradowska-Stolarz A, Wieckiewicz W. Psychosocial aspects of bruxism: The most paramount factor influencing teeth grinding. Biomed Res Int. 2014;2014:469187.
- Ahlberg J, Lobbezoo F, Ahlberg K, Manfredini D, Hublin C, Sinisalo J, et al. Self-reported bruxism mirrors anxiety and stress in adults. Med Oral Patol Oral Cir Bucal. 2013;18(1):e7-11.
- Schecklmann M, Landgrebe M, Langguth B, Group TRIDS. Phenotypic characteristics of hyperacusis in tinnitus. PLoS One. 2014;9(1):e86944.
- Villaume K, Hasson D. Health-relevant personality is associated with sensitivity to sound (hyperacusis). Scand J Psychol. 2017;58(2):158-69.
- Chen YC, Chen GD, Auerbach BD, Manohar S, Radziwon K, Salvi R. Tinnitus and hyperacusis: Contributions of paraflocculus, reticular formation and stress. Hear Res. 2017.
- Chen YC, Li X, Liu L, Wang J, Lu CQ, Yang M, et al. Tinnitus and hyperacusis involve hyperactivity and enhanced connectivity in auditory-limbic-arousal-cerebellar network. Elife. 2015;4:e06576.
- Song JJ, De Ridder D, Weisz N, Schlee W, Van de Heyning P, Vanneste S. Hyperacusis-associated pathological resting-state brain oscillations in the tinnitus brain: A hyperresponsiveness network with paradoxically inactive auditory cortex. Brain Struct Funct. 2014;219(3):1113-28.
- Auerbach BD, Rodrigues PV, Salvi RJ. Central gain control in tinnitus and hyperacusis. Front Neurol. 2014;5:206.
- Gilles A, Goelen S, Van de Heyning P. Tinnitus: A cross-sectional study on the audiologic characteristics. Otol Neurotol. 2014;35(3):401-6.
- Moller AR, Salvi R, De Ridder D, Kleinjung T, Vanneste S. Pathology of Tinnitus and Hyperacusis-Clinical Implications. Biomed Res Int. 2015;2015:608437.
- Juris L, Andersson G, Larsen HC, Ekselius L. Psychiatric comorbidity and personality traits in patients with hyperacusis. Int J Audiol. 2013;52(4):230-5.
- Fioretti AB, Fusetti M, Eibenstein A. Association between sleep disorders, hyperacusis and tinnitus: Evaluation with tinnitus questionnaires. Noise Health. 2013;15(63):91-5.
- Meeus OM, Spaepen M, Ridder DD, Heyning PH. Correlation between hyperacusis measurements in daily ENT practice. Int J Audiol. 2010;49(1):7-13.
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound--questionnaire data, audiometry and classification. Scand Audiol. 1999;28(4):219-30.
- Wa