- Biomedical Research (2007) Volume 18, Issue 1
Smilax calophylla overcomes the effects of adrenalectomy on the testicular 11ß-hydroxysteroid dehydrogenase activity and plasma levels of testosterone in rats
Nuraliza AS1, Khalid BAK2, Hamid A3, Morat PB4 and Nwe KHH31Faculty of Medicine, University Technology MARA, 40450 Shah Alam, Selangor, Malaysia
2School of Medicine and Health Sciences, Monash University Malaysia Campus, 46150 Petaling Jaya, Selangor, Malaysia
3Faculty of Medicine and Health Sciences, University Putra Malaysia, 43400 Serdang, Selangor, Malaysia
4Department of Biomedical Science, Faculty of Allied Health Sciences, Universiti Kebangsaan Malaysia, 50300 Kuala Lumpur, Malaysia
- *Corresponding Author:
- Nuraliza AS
Faculty of Medicine University Technology MARA
40450 Shah Alam, Selangor, Malaysia
E-mail: nuraliza064@salam.uitm.edu.my
Accepted date: June 07 2006
Abstract
Removal of adrenal gland (adrenalectomy) caused profound effects on male reproductive system. Adrenalectomy suppressed testicular 11β-hydroxysteroid dehydrogenase (11β-HSD) activity and plasma levels of testosterone (T) in rats. Our previous study documented that corticosterone-induced inhibition on testicular 11β-HSD activity and plasma levels of T could be reversed by Smilax calophylla, (Akar dawai) water extract (AD). The present study was therefore designed to determine whether the effects of adrenalectomy on the testicular 11β-HSD activity and plasma levels of T could be reversed by AD. Testicular 11β-HSD activity and plasma levels of T were found to be reduced significantly (p<0.001) following adrenalectomy. However, AD administration in the adrenalectomized rats was found to retain the testicular 11β-HSD activity and plasma levels of T as found in controls rats. The present study therefore suggests that AD is able to counteract the effects of adrenalectomy on the testicular 11β-HSD activity and plasma levels of T.
Keywords
Smilax calophylla, adrenalectomy, 11β-HSD, plasma testosterone
Introduction
Long term glucocorticoid deficiency could eventually lead to disturbances in male reproductive function, where low plasma levels of testosterone (T) and delayed spermatogenesis are the subsequent consequences of reduced luteinizing hormone (LH) secretion [1]. Likewise, adrenalectomy evidently suppresses the 11β-hydroxysteroid dehydrogenase (11β-HSD) activity in the testis and liver [2,3] as well as plasma T levels [4].
11β-hydroxysteroid dehydrogenase a ubiquitous enzyme, which is known to be responsible in modulating the glucocorticoid levels in the tissues [5,6]. In the testis, 11β-HSD converts excess corticosterone (CORT) to the inactive form; thereby protects the testis from the adversed effect of CORT especially on T production [7]. Corticosterone, on the other hand has been reported to have a direct inhibitory effect on testicular steroidogenesis [8]. In certain treatments, the oxidative activity of 11β-HSD has been correlated with plasma levels of T [9]. Conversely, suppression of the 11β-HSD activity has been shown to be associated with low plasma levels of T or viceversa [10].
Smilax calophylla (Akar dawai) is found mainly in the northern part of Peninsular Malaysia. The local folks consume decoction of Akar dawai rhizome as a male sexual tonic. Indeed, the Smilax plant has been reported to be a potential aphrodisiac [11]. Studies on some other Smilax plants have documented the steroidal components of the plant rhizomes [12,13] as well as of the various effects of the Smilax plant extract [14,15]. Our previous study revealed that the water extract of Akar dawai rhizome (AD) could overcome the suppressive effect of exogenous CORT on testicular 11β-HSD activity and plasma levels of T in rats, and it is proposed that AD possibly acts by blocking glucocorticoid receptors [16].
Materials and methods
Chemicals
Chemicals used in the enzyme assay such as corticosterone, 11-dehydrocorticosterone, glucose, bovine serum albumin (BSA), and NADP were purchased from Sigma Chemical Co. St. Louis, USA. Bio-Rad Laboratory, CA, USA supplied the dye reagent used. The Coatacount commercial RIA kit for total testosterone was obtained from Diagnostic Products Corp., Los Angeles, CA. Akar dawai water extract was kindly supplied by Prof. Dr. Johari Mohd. Saad research group from Department of Biochemistry, Faculty of Medicine, University of Malaya, Kuala Lumpur.
Animals and treatment
Male Wistar rats of 200-250 g body weight (BW) were purchased from the Animal Unit, Institute of Medical Research, Malaysia. Rats were randomly assigned into three groups: control group, adrenalectomized (ADX) control group and ADX rats treated with AD water extract (8mg/kg BW). Six to twelve rats were assigned into each group. Control rats were given normal saline orally. Water extract of AD was gavaged (0.5ml) daily for seven consecutive days. From the time dependant graph of this herb, we found that AD (8mg/kg BW) for three consecutive days in normal rats could increase plasma T levels [16]. Therefore, in addition to the three experimental groups, six identical rats were subjected to bilateral adrenalectomy to compare the enzyme and hormonal parameters.
Bilateral adrenalectomy was performed as per the reported method [17]. Its completeness was inspected and verified at the time of killing. The rats were housed together in a temperature controlled environment (27-29°C) with 12:12 hour light-dark cycle. Normal rats were given tap water as drinking water whereas 0.9% normal saline was substituted to ADX rats. Animal were sacrificed 24 hours after the last treatment schedule between 8.30 and 9.00 a.m.
The Medical Research and Ethics Committee of the Universiti Kebangsaan Malaysia (UKM) had approved methodology used in the present study.
Assay of 11β -HSD enzyme activities
Testes were removed immediately from the sacrificed animals, dissected and homogenized with Krebs solution containing glucose. The enzyme assay was performed as previously described [9] by incubating 250 μl tissue homogenate with 12nM 3H-B and 200 μM NADP at 37°C for 10 minutes (all steps were carried out on ice unless stated otherwise). The reaction was stopped and steroids were extracted by addition of ethyl acetate. Steroids were then separated by thin layer chromatography (TLC) and identified under ultraviolet light. The radioactivity was determined in β-liquid scintillation counter. 11β-HSD oxidative activity was expressed as percentage conversion of corticosterone to 11-dehydrocorticosterone.
Radioimunoassay (RIA) of plasma testosterone
Animals were anesthetized by diethyl ether and 3-4 ml blood was collected in heparinized tube. Ether was used to minimize marked fluctuation of plasma level of T [18]. Hormone levels in the plasma were analyzed as previously reported [4,7]. Plasma T levels were measured using commercial RIA kit (Coat-a-count, Diagnostic Products Corp. CA). The intra- and interassay variation coefficients of the total T was within 10%.
Statistical analysis
Data were analyzed using Statistix Programme. The data for 11β-HSD oxidative activities were expressed as mean ± standard error of mean (SEM) while for plasma T levels were expressed as mean ± 95% confidence interval (CI). Differences between groups were analyzed by analysis of variance (ANOVA) and student t-test. The differences were considered significant at p<0.05. Statistical analysis was made in accordance with the previous report [19].
Results
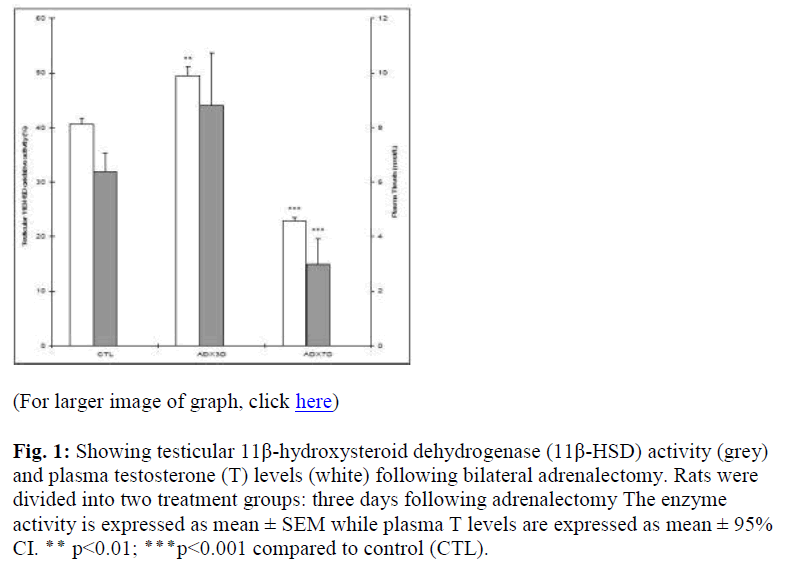
The 11β-HSD activity was significantly higher three days following adrenalectomy (ADX3D), (p<0.01). However, the plasma T levels remained unchanged compared to that of the controls (Fig. 1). Moreover, a significant reduction in 11β-HSD activity (p<0.001) as well as plasma T levels (p<0.001) was recorded following seven days of adrenalec tomy compared to that of the control value (Fig. 1).
Figure 1: Showing testicular 11β-hydroxysteroid dehydrogenase (11β-HSD) activity (grey) and plasma testosterone (T) levels (white) following bilateral adrenalectomy. Rats were divided into two treatment groups: three days following adrenalectomy The enzyme activity is expressed as mean ± SEM while plasma T levels are expressed as mean ± 95% CI. ٭٭ p<0.01; ٭٭٭p<0.001 compared to control (CTL).
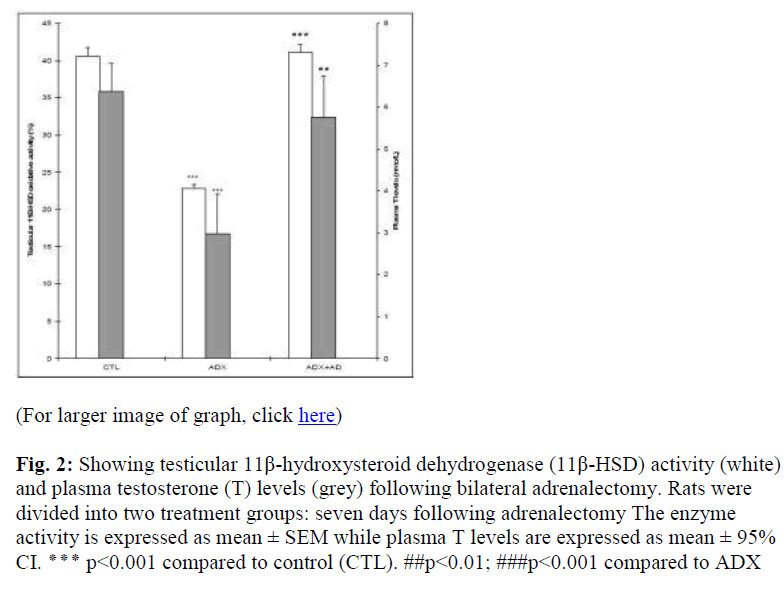
Administration of Akar dawai water extract (ADX+AD), on the other hand, increased the 11β-HSD activities (p<0.001) and plasma T levels (p<0.01) compared to ADX rats (Fig. 2).
Figure 2: Showing testicular 11β-hydroxysteroid dehydrogenase (11β-HSD) activity (white) and plasma testosterone (T) levels (grey) following bilateral adrenalectomy. Rats were divided into two treatment groups: seven days following adrenalectomy The enzyme activity is expressed as mean ± SEM while plasma T levels are expressed as mean ± 95% CI. ٭٭٭ p<0.001 compared to control (CTL). ##p<0.01; ###p<0.001 compared to ADX
Discussion
Adrenalectomy has been reported to reduce luteinizing hormone levels (LH) as well as the levels of plasma T in male rats [1]. We have observed that administration of AD (8mg/kg BW) in rats for three consecutive days elevates plasma T levels, without altering the testicular 11β-HSD activities. On the other hand, administration of AD (8mg/kg BW) for seven days, although significantly decreases the 11β-HSD activity, yet the plasma T levels remain unchanged [16]. Results of our experiment show that three days following bilateral adrenalectomy the 11β-HSD activity has been elevated, however the plasma levels of T remain unaltered. It has been reported that seven day after bilateral adrenalectomy, both the parameters are reduced significantly [4,10].
Treatment of the water extract of AD to adrenalectomized rats has been found to maintain the activity of 11β-HSD and plasma levels of T almost identical to controls. Treatment of AD for seven consecutive days in normal rats has similarly been found to counteract the inhibitory effect of exogenous CORT, on the 11β-HSD activity and plasma levels of T. Infact, AD is proposed to act via glucocorticoid receptor in affecting both parameters [16]. It has been suggested that 11β-HSD activity and plasma T levels regulate each other in certain conditions [9,10]. In conclusion, our finding suggests that water extract of AD is possibly able to overcome the effects of bilateral adrenalectomy on testicular function.
Acknowledgement
We wish to thank Prof. Dr. Johari Mohd. Saad research group from the Faculty of Medicine, University of Malaya for extracting and supplying the Smilax calophylla (AD) water extract. The present research was funded by IRPA research grant 06-02-02-0081. A special thanks to Professor Dr Amar Chatterjee, Faculty of Medicine, UiTM for his critical comments in the preparation of this manuscript.
References
- Lescoat G, Lescoat D, Garnier DH. Influence of adrenalectomy on maturation of gonadotrophin function in the male rat. J Endocrinol. 1982; 95: 1-6.
- Gao HB, Shan LX, Monder C, Hardy MP. Suppression of endogenous corticosterone levels in vivo increases the steroidogenic capacity of purified rat Leydig cells in vitro. Endocrinology. 1996; 137: 1714-1718.
- Nwe KHH, Hamid A, Morat PB, Khalid BAK. Differential regulation of the oxidative 11β-hydroxysteroid dehydrogenase activity in testis and liver. Steroids. 2000 ; 65: 40-45.
- Nwe KHH, Norhazlina AW, Hamid A, Morat PB, Khalid BAK. In vivo effects of stress, ACTH and corticosterone on testicular 11β-hydroxysteroid dehydrogenase oxidative activity in rats and the possible mechanism of action. Exptl Clin Endocrinol Diabetes. 2000; 106: 1-9.
- Phillips DM, Lakshmi V, Monder C. Corticosteroid 11β-dehydrogenase in rat testis. Endocrinology. 1989; 125: 209-216.
- Walker BR, Edwards CRW. 11β-hydroxysteroid dehydrogenase and enzyme-mediated receptor protection : Life after liquorice. Clin Endocrinol. 1991; 35 : 281-289.
- Monder C, Miroff Y, Marandici A, Hardy MP. 11β-hydroxy-steroid dehydrogenase alleviates glucocorticoid-mediated inhibition of steroidogenesis in rat Leydig cells. Endocrinology. 1994; 134: 1199-1204.
- Bambino TH, Hsueh AJW. Direct inhibitory effects of gluco-corticoids upon testicular luteinizing hormone receptor and steroidogenesis in vivo and in vitro. Endocrinology. 1981; 108: 2142-2147.
- Nwe KHH, Hamid A, Norhazlina AW, Khalid BAK, Morat PB. The relationship between plasma testosterone levels and testicular or hepatic 11β-hydroxysteroid dehydrogenase activity in normal rats with various treatments. J Asean Fed Endocrinol Soc. 2000; 18: 6-13.
- Nwe KHH, Morat PB, Hamid A, Fadzilah S, Khalid BAK. Novel effects of deoxycorticosterone on testicular 11β-hydroxysteroid dehydrogenase activity and plasma testosterone levels in normal and adrenalectomized rats. Exptl Clin Endocrinol Diabetes. 1999; 107: 1-7.
- Gimlette JD. A dictionary of Malayan medicine, Oxford University Press, Kuala Lumpur. 1971.
- Bernardo RR, Pinto AV, Parente JP. A steroidal saponins from Smilax officinalis. Phytochemistry. 1996; 43: 465-469.
- Kubo S, Mimaki Y, Sashida Y, Nikaido T, Ohmoto T. Steroidal saponins from the rhizomes of Smilax sieboldii. Phytochemistry. 1992; 31: 2445-2450.
- Caceres A, Lopez BR, Giron MA, Longemann H. Plants used in Guatemala for the treatment of dermatophytic infections. 1. Screening for anti-mycotic activity of 44 plants extracts. J Ethnopharmacol. 1991; 31: 263-276.
- Fukunaga T, Miura T, Furuta K, Kato A. Hypoglycemic effect of the rhizomes of Smilax glabra in normal and diabetic mice. Biol Pharma Bull. 1997; 20: 44-46.
- Nuraliza AS, Nwe KHH, Morat PB, Hamid A, Khalid BAK. The effects of Smilax calophylla on testicular 11β-hydroxysteroid dehydrogenase activity and plasma testosterone levels in rats. Biomedical Research. 2006; 17: 55-59.
- Khalid BAK, Paden M, Zainuddin M. The effects of naloxone, dexamethasone, deoxycorti-costerone and 17-hydroxyprogesterone on blood pressure responses of normal and adrenalectomized rats during hypovolemic shock. Clin Exp Pharm Physio. 1987; 14:111-117.
- Lamming GE. Marshall’s Physiology of Reproduction. 4th Ed. Churchill Livingstone., Longman Group, United Kingdom. 1990
- Pipkin FB. .Medical Statistics Made Easy. Churchill Livingstone, Edinburgh, UK. 1984.‘