Commentary - Biomedical Research (2017) Volume 28, Issue 10
Simultaneous determination of some heavy metals in nail samples of Saudi Arabian smokers by inductive coupled plasma mass spectrometry
AL-Ramadi MA1, N. A. AL-Askar1 and Mostafa GAE2,3*
1Forensic Chemistry Department, Naïf Arab University for Security Science, Saudi Arabia
2Pharmaceutical Chemistry Department, College of Pharmacy, King Saud University, P.O. Box 2457, Riyadh, Saudi Arabia
3Micro-analytical Laboratory, Applied Organic Chemistry Department, National Research Center, Dokki, Cairo, Egypt
- *Corresponding Author:
- Mostafa GAE
Pharmaceutical Chemistry Department
College of Pharmacy
King Saud University, Saudi Arabia
E-mail: gamal_most@yahoo.com; gmostafa@ksu.edu.sa
Accepted date: March 4, 2017
Abstract
In the present study, the levels of some heavy elements in the nails of smokers and non-smokers were estimated to study the relationship between smoking and the presence of toxic elements in Saudi Arabia population. Nail samples were collected from cigarette smokers, tobacco pipe smokers, and non-smoker volunteers. The concentration of toxic metals was measured using inductively coupled plasma-mass spectrometry after microwave acid digestion. The validity of the proposed method was investigated by analysing metals ions in certified water samples. The average concentration of Nickel (Ni), Chromium (Cr), Zinc (Zn), Cadmium (Cd), Lead (Pb), and mercury (Hg) was 10.68 ± 7.96, 70.89 ± 34.58, 89.35 ± 27.91, 0.08 ± 0.08, 13.11 ± 19.34, and 1.43 ± 1.19 in cigarette smoker, 12.73 ± 9.12, 62.01 ± 22.51, 85.5 ± 15.92, 3.64 ± 4.48, 0.09 ± 0.11, and 1.84 ± 1.44 in tobacco pipe smoker, and 5.21 ± 4.94, 42.36 ± 16.84, 65.02 ± 28.89, 1.56 ± 0.99, 0.06 ± 0.05, and 0.81 ± 0.58 in non-smoker samples, respectively. Statistical analysis indicated a significant correlation between the concentration of these metals and smoking (either cigarette or tobacco pipe), compared with non-smokers. Moreover, there was a correlation between the concentration of these elements and the period of smoking.
Keywords
Toxic metals, Nail samples, Smokers, Non-smokers, ICP-MS
Introduction
Tobacco use is an important factor in many chronic diseases including cancer, lung disease, and cardiovascular disease, despite the numerous regulations that restrict its use worldwide [1]. Many carcinogenic materials, including the toxic elements found in tobacco and tobacco products [2], reach the bloodstream by smoking and accumulate in different organs [3]. Smoking is an important source of exposure to toxic elements, which can cause physiological disorders [4-6]. Permanent exposure to toxic elements can cause many diseases. For example, Cadmium (Cd) causes kidney failure [7], Lead (Pb) causes problems in the heart, blood vessels, and nerves [8], and Nickel (Ni) causes cardiovascular and kidney diseases [9]. Ni forms carcinogenic compounds [2] by reacting with carbon monoxide that is produced from tobacco smoking. According to the Agency for Toxic Substances and Disease Registry (ATSDR) [10], Cd, Chromium (Cr), Mercury (Hg), and Pb are among the top 10 toxic metals in the priority list of hazardous substances. Zinc (Zn) is a key element within the human body and an essential cofactor for various enzymes [11]. Zn deficiency is associated with increased Cd, as a result of the hostile relationship between Zn and Cd [12].
Many studies have estimated the concentrations of heavy metals and toxic elements, and the relationship between the presence of these elements and smoking habits, to clarify whether smoking is a source of these metals. Previously, in a study by Hussain et al. [13], the concentration of heavy metals in the nails of smokers was compared with those in nails of non-smokers by using atomic absorption spectrometry.
In Maiduguri, Nigeria, a study was conducted to determine the concentrations of Iron (Fe), Cr, Arsenic (As), Cd, Ni, and Pb using atomic absorption spectrophotometry [14]. The hair and nail samples of workers employed in wineries and in metal workshops were collected. They found that the concentrations of Cd, Pb, Cr, Fe, Ni, Copper (Cu), and Zn were higher in the samples from smokers compared with non-smokers.
Another study was conducted in Pakistan for the determination of Copper (Cu), Cd, and Pb in the hair and nail samples of workers from various industrial environments as well as smokers were estimated using a flame atomic absorption technique [15]. High concentrations of these metals were found in both groups. Similarly study by Bruno et al. [16] used inductively coupled plasma mass spectrometry (ICP/MS) for the simultaneous determination of Cd, Cu, Mn, Ni, Pb, and Zn in nail samples and compared the results with electro-thermal atomic absorption spectrometry technique.
The detection of toxic metals in biological samples for human clinical examination is important which use as a marker for many diseases [4-9]. The present study aims to estimate the level of toxic elements in nail the samples of smokers to study the relationship between human habits, such as smoking, as well as the smoking period, and the presence of toxic elements in the human body.
Nail samples are advantageous over other biological samples, because elements accumulate over a long period of time without any changes. Nail samples do not require any special conditions for storage, and the analysis can be done safely without any loss of these elements. In addition, the collection of the samples is very easy [17,18].
The aim of the present work was to develop a sensitive method for determination of some heavy metals in nails of smokers and non-smokers in Saudi Arabia population using inductive coupled plasma mass spectrometry.
Experimental
Chemical and reagents
Nitric acid (purity: 69 -71%), HCl (36-38%), and hydrogen peroxide (H2O2; 35%) were purchased from (BDH Poole, England) Ni (N9300177), Cr (N9303736), Zn (N9300168), Pb (N9300128), Cd (N9300176), Hg (N9300133), were purchased from Perkin Elmer, USA . Triton X-100, acetone, rhodium (Rh) (Internal standard) (04026) were obtained from Sigma Aldrich, Germany.
Apparatus
An inductively coupled plasma mass spectrometer (NexION 300D; Perkin Elmer, New York, USA) was used. The instrumental conditions are summarized in Table 1. Deionized water was obtained using a water purification system (PURELABE OPTION-Q15; ELGA Lab Water, United Kingdom). A Milestone microwave digestion system (Ethos One; Sorisole, Italy) was used.
| RF power | 1250W |
| Nebulizer gas glow | 0.92 L/min |
| Lens voltage | 9.25 V |
| Analog stage voltage | -1762.5 V |
| Pulse stage voltage | 1050 V |
| Replicates per sample | 3 |
| Reading/Replicates | 20 |
| Scan mode | Peak hopping |
| Dwell time | 40 ms |
| Integration | 1200 ms |
| Internal standard | Rhodium 500 ppb |
Table 1. Measurement conditions for ICP-MS.
Sample collection and pre-treatment
Nails were collected from volunteers who smoked cigarettes or tobacco pipes or were non-smokers (control) over the course of one year (January 2014 to January 2015). The study was conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee of King Saud University, Riyadh, Saudi Arabia [16]. The samples were classified according the smoking period, and individually stored in plastic bags with custom date entries. Each sample bag was labeled accordingly: sample number, age, and period of smoking.). Samples were collected from volunteers who smoked cigarettes (n=30), tobacco pipes (n=30), and nonsmokers (n=15).
Preparation of standard solutions samples
Two working solutions (100 and 10 μg/ml) of each element (Ni, Cr, Zn, Cd, Pb, Hg and Rh) were prepared by suitable dilution of each standard with de-ionized water.
Sample treatment and digestion
The sample was digested via acid digestion (mixture of concentrated nitric acid and perchloric acid) in a microwave. The nail samples were washed with a mixture of Triton X-100 and acetone according to the method described by Gammelgaard et al. [19], before digestion. After the drying process, nail samples were weighed and (0.5 g) placed in Teflon containers to be microwaved, and then 10 ml of a mixture containing concentrated nitric acid and perchloric acid (1:6) was added. The microwave operation was modified as per a report by the US Environmental Protection Organization (USEPA) [20]. The solution was then transferred to a 50 ml measuring flask and completed to the mark with deionized water, and an aliquot was inserted into the cell for measurement using ICP/MS. A blank sample, which underwent the same conditions, containing 0.5 ml of deionized water was also used.
Validation of the proposed method
The suggested method was validated using certified reference material (KATS No. 2008-242) [21] containing fixed concentrations of the metal ions. The validation parameters included linearity, sensitivity, selectivity, precision, and accuracy. ICP/MS, after microwave acid digestion, was conducted for the simultaneous determination of toxic elements (Ni, Cr, Zn, Cd, Pb, and Hg) in the nail samples. The procedure was optimized to ensure linearity, accuracy, and precision. Calibration curves were in the concentration range of 10-100 μg/l for Ni, Cr, Zn, Cd and Pb, while Hg was in the range of 100-300 μg/l and Rh was used as the internal standard.
Statistical analysis
The volunteers were divided into three groups: cigarette smokers, tobacco pipe smokers, and non-smokers (control). Results are presented as mean ± Standard Deviation (SD). Data were analysed using a one-way analysis of variance by using Microsoft excel Window 2010 and the Statistical Package for the Social Analysis (SPSS; version 16) using parametric study . Values<0.05 were considered statistically significant. The concentrations of six elements (Ni, Cr, Zn, Pb, Cd, and Hg) were analysed and the group type (cigarette, tobacco pipe, and control) was used as the independent variable. The average concentration and smoking period were also examined [22].
Results
To investigate the validity of the proposed method, certified water samples were analysed using ICP/MS. The average recovery was 103.13%, 96.33%, 96.55%, 98.90%, 97.37% and 95.0% and the relative standard deviation was 1.7%, 2.8%, 3.1%, 1.5%, 4.6% and 6.2% for Ni, Cr, Zn, Pb, Cd, and Hg, respectively. Results are summarized in Table 2.
| Element | Certified value (µg/L) | Found (µg/L) | Recovery (%)* | SD | RSD (%) |
|---|---|---|---|---|---|
| Ni | 58.1 | 59.92 | 103.1 | 1.02 | 1.7 |
| Cr | 18.5 | 17.85 | 96.33 | 0.5 | 2.8 |
| Zn | 72.48 | 69.98 | 96.55 | 2.15 | 3.1 |
| Pb | 18.15 | 17.95 | 98.89 | 0.26 | 1.5 |
| Cd | 6.47 | 6.3 | 97.37 | 0.29 | 4.6 |
| Hg | 40 | 38 | 95 | 0.002 | 6.2 |
Table 2. Determination of toxic metals in certified sample [21] using the proposed ICP/MS method.
Precision and repeatability
The intra- and inter-day precision of the proposed method was assessed. Precision is expressed as the Relative Standard Deviation (RSD (%)) and the accuracy is expressed by recovery (%). The average recovery was 96.55, 96.33, 96.55, 98.90, 97.37, and 95.0% for Ni, Cr, Zn, Pb, Cd, and Hg, respectively. The RSD% was 1.7, 2.8, 3.1, 1.5, 4.6, and 6.2% for Ni, Cr, Zn, Pb, Cd, and Hg, respectively. These results are summarized in Table 2.
Determination of toxic elements in nail samples
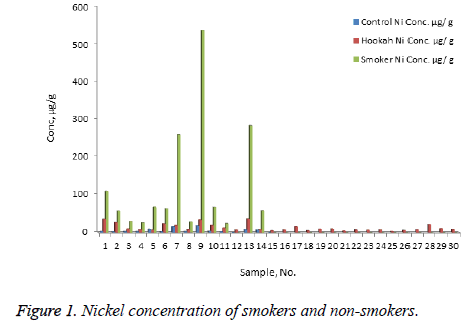
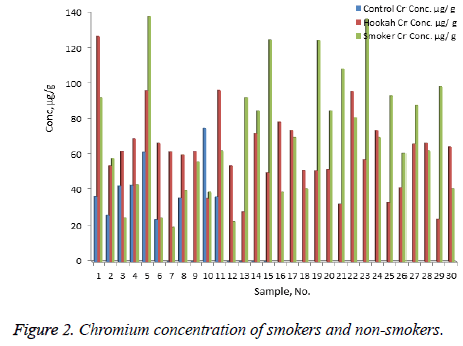
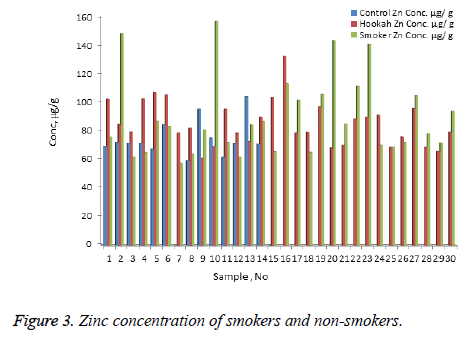
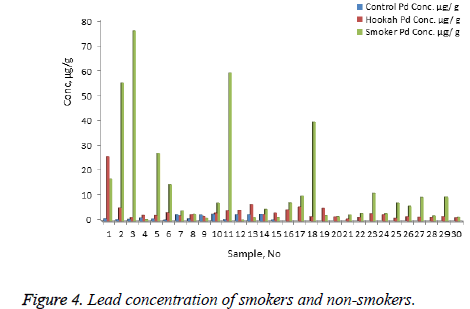
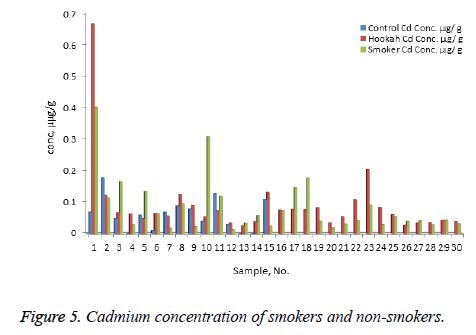
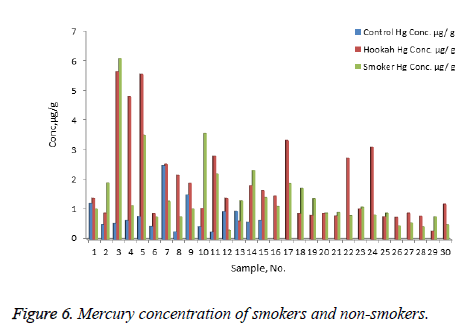
The concentration of Ni, Cr, Zn, Cd, Pb, and Hg were quantified using the validity ICP-MS method for nail samples of the study volunteers. The concentration of Ni (range: nonsmokers 0-16.54 μg/g, cigarette smokers, 1-30.17 μg/g, tobacco pipe smokers, 3.6-35.75 μg/g), Cr (range: non-smokers 23.78-75.25 μg/g, cigarette smokers, 19.66-138.41 μg/g, tobacco pipe smokers, 23.96-127.30 μg/g), Zn (range: nonsmokers 0.00-104.29 μg/g, cigarette smokers, 57.88-157.30 μg/g, tobacco smokers, 60.93-132.99 μg/g), Pb (range: nonsmokers 0.54-2.91 μg/g, cigarette smokers, 0.56-76.34 μg/g, tobacco smokers, 0.87-26.04 μg/g) Cd (range: non-smokers 0-0.18 μg/g, cigarette smokers, 0.01-0.41 μg/g, tobacco smokers, 0.03-0.67 μg/g) and mercury ( range: non-smokers 0.25-2.50 μg/g, cigarette smokers, 0.32-6.12 μg/g, tobacco pipe smokers, 0.29-5.67 μg/g) were recorded . The mean concentrations of Ni in the non-smoker, cigarette smoker, and tobacco pipe smoker groups (Table 3) were determined as 5.21, 10.68, and 12.73 μg/g, respectively (Figure 1). The mean concentration of Cr in the non-smoker, cigarette smoker, and tobacco pipe smoker groups (Table 3) were determined as 42.36, 70.89, and 62.01 μg/g, respectively (Figure 2). The mean concentrations of Zn in the non-smoker, cigarette smoker, and tobacco pipe smoker groups (Table 3) were determined as 65.02 μg/g, 85.5 μg/g, and 89.35 μg/g, respectively (Figure 3). The mean concentrations of Pb in the non-smoker, cigarette smoker, and tobacco pipe smoker groups (Table 3) were 1.57 μg/g, 13.11 μg/g and 3.64 μg/g, respectively (Figure 4). The mean concentrations of Cd in the non-smoker, cigarette smoker, and tobacco pipe smoker groups (Table 3) were 0.06 μg/g, 0.08 μg/g and 0.09 μg/g, respectively (Figure 5). The mean concentrations of Hg in the non-smokers, cigarette smokers and tobacco pipe smokers (Table 3) were 0.43, 1.43 and 1.84 μg/g, respectively (Figure 6). The values obtained in our study are higher than those previously reported (0.23 μg/g).
| Element | Group | Sample no | Average* | Standard division | Low value | Higher value | Error in standard division |
|---|---|---|---|---|---|---|---|
| Ni | Cigarette | 30 | 10.68 | 7.96 | 1.45 | 30.17 | 1.45 |
| Hookah | 30 | 12.73 | 9.12 | 1.66 | 35.75 | 1.66 | |
| Control | 15 | 5.21 | 4.94 | 1.72 | 16.45 | 1.77 | |
| Cr | Cigarette | 30 | 70.89 | 34.58 | 19.66 | 138.41 | 6.31 |
| Hookah | 30 | 62 | 22.51 | 23.96 | 127.3 | 4.11 | |
| Control | 15 | 42.36 | 16.48 | 23.78 | 75.25 | 5.49 | |
| Zn | Cigarette | 30 | 89.35 | 27.91 | 57.88 | 157.3 | 5.09 |
| Hookah | 30 | 85.5 | 15.92 | 60.93 | 132.93 | 2.9 | |
| Control | 15 | 65.02 | 28.89 | 0 | 104.29 | 7.45 | |
| Pb | Cigarette | 30 | 13.11 | 19.34 | 0.56 | 76.33 | 3.53 |
| Hookah | 30 | 3.63 | 4.48 | 0.87 | 26.04 | 0.81 | |
| Control | 15 | 1.56 | 0.99 | 0.54 | 2.91 | 0.25 | |
| Cd | Cigarette | 30 | 0.08 | 0.08 | 0.01 | 0.41 | 0.01 |
| Hookah | 30 | 0.09 | 0.11 | 0.03 | 0.67 | 0.02 | |
| Control | 15 | 0.66 | 0.05 | 0 | 0.18 | 0.01 | |
| Hg | Cigarette | 30 | 1.43 | 1.19 | 0.32 | 6.12 | 0.21 |
| Hookah | 30 | 1.84 | 1.44 | 0.29 | 5.67 | 0.26 | |
| Control | 15 | 0.81 | 0.58 | 0.25 | 2.5 | 0.15 | |
| *Average of six determinations | |||||||
Table 3. Mean concentration of nickel, chromium, zinc, lead, cadmium and mercury in nail samples of smokers (cigarette and hookah) and nonsmokers.
Statistical analysis
Statistical analysis was conducted to analyse the link between smoking and the presence of toxic metals. In addition, we examined the relationship between the exposure by these elements and smoking and/or the smoking period. Comparison between concentration of toxic elements between smoker and non-smokers samples, F test was carried out , the result show that the F test was 4.45, 3.66, 5.40, 5.98, 0.51 and 3.56 with significant values of 0.015, 0.031, 0.007, 0.004, 0.61 and 0.033 for Ni, Cr, Zn, Pb, Cd and Hg, respectively. The statistical differences were observed for Ni, Cr, Zn, Pb and Hg and no for Cd. Results are shown in Table 4. Moreover, the significance of the smoking period was analysed. The results are shown in Table 5. The F test values were 1.45, 1.97, 1.34, 2.63, 1.66, and 1.84, with P values of 0.162, 0.038, 0.218, 0.006, 0.093, and 0.055, for Ni, Cr, Zn, Pb, Cd, and Hg, respectively. The smoking period was considered statistically significant for Cr and Pb but not for Ni, Zn, Cd, or Hg.
| Element | F test | Significance |
|---|---|---|
| Ni | 4.45 | 0.015 |
| Cr | 3.66 | 0.031 |
| Zn | 5.4 | 0.007 |
| Pb | 5.98 | 0.004 |
| Cd | 0.51 | 0.61 |
| Hg | 3.56 | 0.033 |
Table 4. One-way ANOVA test between average concentration of metals and smoking.
| Element | F test | Significant |
|---|---|---|
| Ni | 1.45 | 0.162 |
| Cr | 1.97 | 0.038 |
| Zn | 1.34 | 0.218 |
| Pb | 2.63 | 0.006 |
| Cd | 1.66 | 0.093 |
| Hg | 1.84 | 0.055 |
Table 5. One-way ANOVA test between average concentration and smoking period.
The regression equations obtained from the calibration graph for Ni, Cr, Zn, Cd, Pb, and Hg was: y=0.0084x+0.0007, y=0.0085x-0.0074, y=0.0084x+0.0025, y=0.0089x+0.0011, y=0.0088x-0.0021 and y=0.0029x+0.0048, respectively, and the correlation coefficient (r2) were 0.999 for all the elements. To investigate the validity of the proposed method, certified water samples were analysed using the proposed ICP/MS.
Discussion
Smoking is one of the main sources of exposure to toxic elements, which can cause many diseases [7-9].
Overall, the concentrations of toxic metals were higher in smokers compared with those in non-smokers, and this is consistent with the literature [24-27]. The levels of Ni are higher than previously reported data by Gautam et al. [24], who found an average of 3.89 μg/g. Bruno et al. [16] and Rodushkiu and Axelsson [25] also found in concentration range of 0.41-3.22 μg/g and 0.14-6.96 μg/g, respectively. Our values of Cr are similar to those reported in the literature, e.g. 102.64 mg/g by Salman et al. [26] and 62.01 mg/g by Gomez et al. [27]. The values of Zn from the cigarette and tobacco pipe smoker groups are similar to previous data from Gautam et al. [24], who found an average of 97.7 μg/g, however, they were lower than the concentrations reported by Bruno et al. [16], who noted an average of 73-104 μg/g. In case of lead our concentrations are lower than those reported previously by Salman et al. [26], who reported an average of 49.86 μg/g, and Gomez et al. [27] who reported an average of 56.83 μg/g. However, these concentrations are higher than those reported by Rodushkiu and Axelsson [25] who reported in the range of 0.27-4.75 μg/g and Bruno et al. [16] who found an average of 0.1-0.83 μg/g. Our concentration of Pb are lower than those previously reported by Gautam et al. [24] and Gomez et al [27] 0.32, and 0.75 μg/g, respectively. However, our concentration is much higher than those reported (0.014 μg/g) [28]. In case of mercury , our results are higher than the concentration that have been detected in study that carried out in India [24], which has been monitoring the average concentrations in nail samples of (0.32 μg/g) and less than the concentration which carried out by Gomez et al. [27] in nail samples which 0.06 μg/g. Our studied concentration was high compared with the study which was carried out in Riyadh which is 0.014 μg/g [24] and 0.06 μg/g [27].
Conclusion
The present study ICP/MS was used to determine the concentration of toxic metals in the nail samples of cigarette smokers, tobacco pipe smokers and no-smokers. The levels of the investigated elements in the nails of smokers were high compared with those in non-smokers, and this was statistically significant, with regard to the smoking habit, for Pb, Cr, Ni, Zn, and Hg but not for Cd. For the smoking period, the levels of Cr and Pb were significantly different but the levels of Ni, Zn, Cd, or Hg were not. This study shows that the presence of toxic metals in biological sample (nail) are consider as marker for many diseases.
Acknowledgements
The author extend their appreciation to the Research Center of the college of pharmacy, King Saud university, and the Dean of Scientific Research at King Saud University for funding this work.
References
- Who EC. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157.
- Chiba M, Masironi R. Toxic and trace elements in tobacco and tobacco smoke. Bull World Health Organ 1992; 70: 269-275.
- Reilly C. Metal contamination of food: its significance for food quality and human health. John Wiley 2008.
- Kazi TG, Jalbani N, Kazi N, Jamali MK, Arain MB, Afridi HI, Kandhro A, Pirzado Z. Evaluation of toxic metals in blood and urine samples of chronic renal failure patients, before and after dialysis. Renal Failure 2008; 30: 737-745
- Kazi T, Memon A, Afridi H, Jamali M, Arain M, Jalbani N, Sarfraz R. Determination of cadmium in whole blood and scalp hair samples of Pakistani male lung cancer patients by electrothermal atomic absorption spectrometer. Sci Total Env 2008; 389: 270-276.
- Fitzpatrick TM, Blair EA. Upper airway complications of smoking. Clin Chest Med 2000; 21: 147-157.
- Rathore B, Paul B, Chaudhury BP, Saxena AK, Sahu AP, Gupta YK. Comparative studies of different organs of Nyctanthes arbortristis in modulation of cytokines in murine model of arthritis. Biomed Env Sci 2007; 20: 154.
- Skerfving S, Gerhardsson L, Schutz A, Stromberg U. Lead biological monitoring of exposure and effects. J Trace Elements Exp Med 1998; 11: 289-301.
- Scansetti G, Maina G, Botta GC, Bambace P, Spinelli P. Exposure to cobalt and nickel in the hard-metal production industry. Int Arch Occup Env Health 1998; 71: 60-63.
- ATSDR. A Priority List of Hazardous Substances. Agency for Toxic Substances and Disease Registry 2007.
- Soylk M, Kirnap M. Serum copper and zinc concentrations of patients with rheumatoid arthritis from Kayseri-Turkey. Fresenius Environmental Bulletin 2001; 10: 409-410.
- Kazi TG, Afridi HI, Jamali MK, Arain MB, Jalbani N, Syed N. Evaluation of zinc status in whole blood and scalp hair of female cancer patients. Clinica Chimica Acta 2007, 379: 66-70.
- Bukhari IH, Rasul N, Kausar S, Naqvi SAR, Ali Z, Riaz M. Comparative studies of ni, cd, mn, co, pb, cr and zn in hair, nail and plasma of smokers and non-smokers subjects of Sargodha zone. IJCBS 2013; 4: 28-37.
- Shan U, Ikram N. Heavy metals in human scalp hair and nail samples from Pakistan: influence of working and smoking habits. Int J Chem Biochem Sci 2012; 1: 54-58.
- Mortada WI, Sobh MA, El-Defrawy MM. The exposure to cadmium, lead and mercury from smoking and its impact on renal integrity. Med Sci Monit 2004; 10: 112-116.
- Batista BL, Rodrigues JL, Nunes JA, Tormen L, Curtius AJ, Barbosa F. Simultaneous determination of Cd, Cu, Mn, Ni, Pb and Zn in nail samples by inductively coupled plasma mass spectrometry (ICP-MS) after tetramethylammonium hydroxide solubilization at room temperature: comparison with ETAAS. Talanta 2008; 76: 575-579.
- Barbosa F, Tanus-Santos JE, Gerlach RF, Parsons PJ. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Env Health Perspect 2005; 1669-1674.
- Golasik M, Jawien W, Przybylowicz A, Szyfter W, Herman M, Golusinski W, Florek E, Piekoszewski W. Classification models based on the level of metals in hair and nails of laryngeal cancer patients: diagnosis support or rather speculation. Metallomics 2015; 7: 455-465.
- Gammelgaard B, Peters K, Menne T. Reference values for the nickel concentration in human finger nails. J Trace Elem Electr Health Dis 1991; 5: 121-123.
- Schweitzer L, Cornett C. Determination of heavy metals in whole blood using inductively-coupled plasma-mass spectrometry: A comparison of microwave and dilution techniques. The Big M 2008, 4: 75-83.
- Mackey E, Christopher S, Lindstrom R, Long S, Marlow A, Murphy K, Paul R, Popelka-Filcoff R, Rabb S, Sieber J, Certification of three NIST renewal soil standard reference materials for element content: SRM 2709a San Joaquin Soil, SRM 2710a Montana Soil I, and SRM 2711a Montana Soil II. NIST Special Publication 2010; 260: 172.
- Armitage P, Berry G, Matthews JN. Statistical methods in medical research. John Wiley Sons 2008.
- Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry. Pearson Education 2005.
- Samanta G, Sharma R, Roychowdhury T, Chakraborti D. Arsenic and other elements in hair, nails, and skin-scales of arsenic victims in West Bengal, India. Sci Total Env 2004; 326: 33-47.
- Rodushkin I, Axelsson M D. Application of double focusing sector field ICP-MS for multielemental characterization of human hair and nails. Part II. A study of the inhabitants of northern Sweden. Sci Total Env 2000; 262: 21-36.
- Salma M, Rehman R, Anwar J, Mahmud T. Statistical analysis of selected heavy metals by icpoes in hair and nails of cancer and diabetic patients of Pakistan. Electr J Env Agr Food Chem 2012; 11: 163-171.
- Gomez-Aracena J, Riemersma RA, Gutierrez-Bedmar M, Bode P, Kark JD, Garcia-Rodriguez A, Gorgojo L, Vant Veer P, Fernandez-Crehuet J, Kok FJ. Nail cerium levels and risk of a first acute myocardial infarction: The EURAMIC and heavy metals study. Chemosphere 2006; 64: 112-120.
- Hashem A , Abed K. Aluminium cadmium and microorganisms in female hair and nails from Riyadh, Saudi Arabia. J Med Sci 2007; 7: 263-266.