- Biomedical Research (2014) Volume 25, Issue 4
Simultaneous determination of chlorogenic and isochlorogenic acid contents in Xiao'er Yanbian granule by HPLC.
Chunyan Liu*, Yongfeng Li, Ru Bao, Ying ChenDepartment of Pediatrics, The Second Affiliated Hospital of Zhenghzou University, Zhengzhou, 450014 China
- *Corresponding Author:
- Chunyan Liu
Department of Pediatrics
The Second Affiliated Hospital of Zhenghzou University
Zhengzhou, 450014 China
Accepted August 01 2014
Abstract
The contents of chlorogenic acid and isochlorogenic acid A in Xiao'er Yanbian Granule were determined simultaneously by Reversed-Phase HPLC with acetonitrile-methanol-0.1% phosphoric acid (20:6.5:73.5) as the mobile phase. Investigation of the injection amounts of the two types of chlorogenic acids by the methodology revealed that the injection amounts were in good linearity with the peak areas at a range of chlorogenic acid injection amount of 0.050~1.250 μg, and a isochlorogenic acid A injection amount range of 0.025~0.625 μg. The average recovery (n = 9) was 98.59% for chlorogenic acid, and 100.53% for isochlorogenic acid A, with RSDs of 0.42% and 1.17%, respectively. The contents of chlorogenic acid and isochlorogenic acid A in Xiao'er Yanbian Granule were determined to be 0.78 mg/g and 0.15 mg/g, respectively, this quantification method can be used for the comprehensive evaluation of the quality of Xiao'er Yanbian Granule.
Keywords
Xiao'er Yanbian Granule, chlorogenic acid, isochlorogenic acid A, HPLC
Introduction
Xiao'er Yanbian Granule is composed of eight TCM materials, namely Flos Lonicerae, Rhizoma Belamcandae, Radix Tinosporae, Radix Platycodi, Radix Scrophulariae, Radix Ophiopogonis, and Calculus Bovis Artifactus, which is usually used for swollen sore throat, oral erosion, pharyngitis, laryngitis, tonsillitis and other diseases [1]. The drug has been favored by the general public for its excellent efficacy in treatment of pediatric upper respiratory infections without any side effects. China now has nearly a hundred pharmaceutical companies; which manufacture Xiao'er Yanbian Granule, with a very good sale. To improve the quality of Xiao'er Yanbian Granule, enhance its efficacy and ensure its safety, the improvement of the quality control of Xiao'er Yanbian Granule is vitally important.
Flos Lonicerae is one of the most important medicinal materials in Xiao'er Yanbian Granule, which plays a leading role in terms of efficacy. Major active constituents in Flos Lonicerae include chlorogenic acids, isochlorogenic acids and flavonoids [2-6] of which; chlorogenic acid content is the highest that can reach up to 5.19% [2]; followed by isochlorogenic acid, which can be as high as 70% of that of the chlorogenic acid [7]. All the constituents have quite strong anti-bacterial, anti-inflammatory, anti-oxidant, antipyretic and analgesic effects [8-10]. Therefore, it is reasonable to take Flos Lonicerae as the medicinal material used for the quality control of Xiao'er Yanbian Granule, in the 2010 Edition of Chinese Pharmacopoeia, where only the determination of chlorogenic acid content is listed under the article of quantitative determination of Xiao'er Yanbian Granule [11]. For a more scientific and comprehensive quality control of the preparation, multi-index evaluation method was adopted, in accordance with the "Measures for the Administration of Drug Registration of PRC" and relevant technical guidelines [12-13]. We established a HPLC method for the quantification of the active constituents in Flos Lonicerae [14-18]. This study is the first to simultaneously determine the chlorogenic acid and ischlorogenic acid A contents in Xiao'er Yanbian Granule using the ordinary isocratic elution and reversed-phase column with acetonitrilemethanol- 0.1% phosphoric acid as the mobile phase. The results demonstrate that the method is simple, fast and practical.
Material
Materials
Xiao'er Yanbian Granule samples (batch number: 140109, 140110, 140111, 140112, 140113, 140114) and the eight kinds of medicinal materials in the preparation were pro vided by Beijing Tong Ren Tang Pharmaceutical Co., Ltd.; chlorogenic acid reference substance (batch number: 1120136-20140130) and isochlorogenic acid A reference substance (batch number: 1120136-20140231) were purchased from Nanjing Tianqi Biotechnology Co., Ltd., the purities of reference substances were determined to be over 99.8% by normalization method.
Instruments
Agilent 1100 HPLC (G1379A degasser; G1311A quaternary pump; G1314A UVD detector; G132813 sample injector; Agilent 1100 chromatography workstation). Dikma diamonsil column (5 μm, 4.6 mm×150 mm).
Reagents
Reagents used in the mobile phase were chromatographically pure reagents; all the other reagents were of analytical grade.
Methods and Results
Chromatographic conditions
Column: octadecylsilane bonded silica as the filler; mobile phase: acetonitrile-methanol-0.1% phosphoric acid (20:6.5:73.5); detection wavelength: 326 nm; column temperature: 25°C; volume flow rate: 1.0 mL/min; number of theoretical plates: should not be less than 2,000 calculated based on chlorogenic acid peak.
Preparation of reference solutions
Appropriate amounts of chlorogenic acid and chlorogenic acid A reference substances were accurately weighed, placed in brown volumetric flasks separately, and added with methanol to prepare solutions containing 40 μg of chlorogenic acid and 25 μg of chlorogenic acid A per 1 mL A as the reference solutions.
Preparation of test solution
0.5 g of Xiao'er Yanbian Granule was accurately weighed, placed in a stoppered conical flask, added precisely with 25 mL of 70% methanol, stoppered tightly, and weighed, the Xiao'er Yanbian Granule was then ultrasonicated (power 250 W, frequency 33 kHz) for 30 min and 10 min, allowed to cool, and weighed again. After the lost mass was complemented with 70% methanol, the solution was shaken well, filtered by paper, the subsequent filtrate was filtered with 0.45 μm microporous membrane, and the resulting filtrate was collected and used as the test solution.
Preparation of blank sample
Sample not containing Flos Lonicerae was taken, and prepared into the blank sample solution as per the methods under the "Preparation of test solution".
Determination of samples
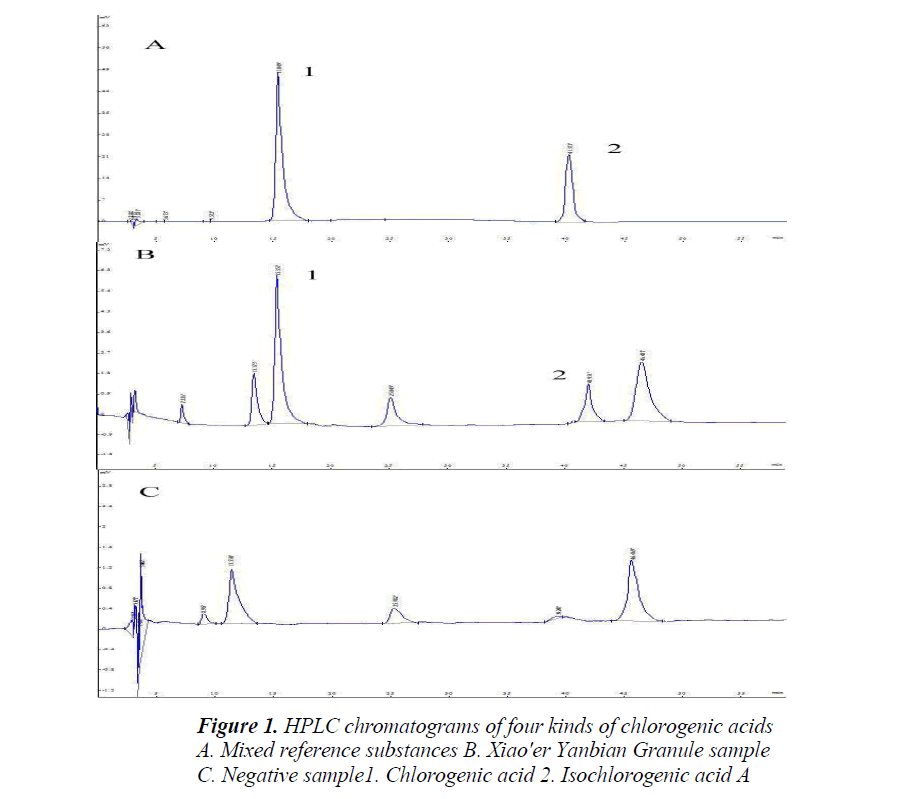
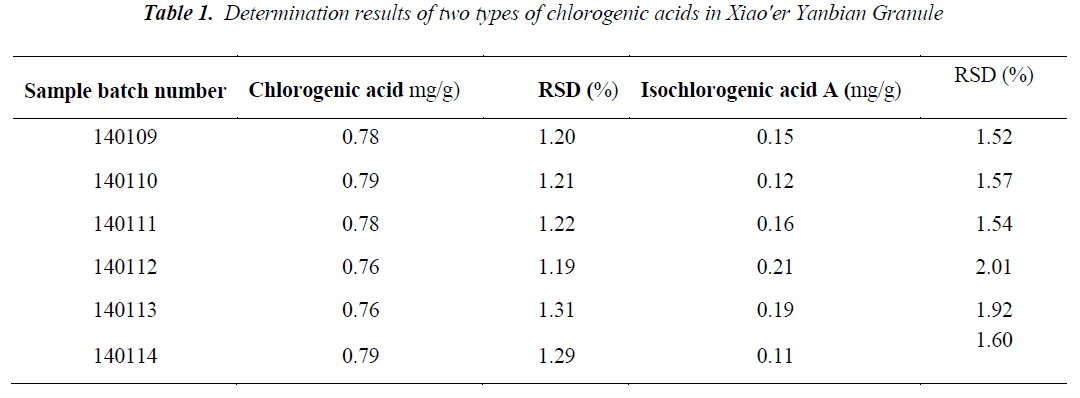
Each 10 μL of the above reference, test and blank solutions were taken, injected into the HPLC, the results indicated that the blank sample was free of interference, the specificity of quantitative determination is shown in Fig. 1; mass fraction was calculated by one point external standard method, the sample determination results are shown in Table 1.
Determination of detection wavelength
Chlorogenic and isochlorogenic acids had the basic parent structure, which were both esters formed between caffeoyl and quinic acid in different locations with different number of caffeoyl [19], so their spectral scan results were consistent, with maximum absorptions both at (326±2) nm, so the detection wavelength was set at 326 nm.
Linearity range
1, 5, 10, 20 and 25 μL of 0.050 mg/ml chlorogenic acid solutions and 0.025 mg/ml isochlorogenic acid A solutions were taken separately, and injected into the HPLC for determination of peak area. Standard curves were plotted with the peak area as the ordinate, and the injection volume as the abscissa. The results revealed that the injection volume was in a good linearity with the peak area at a chlorogenic acid concentration range of 0.05~1.25 μg/ml, and a isochlorogenic acid A concentration range of 0.02~0.625 μg/ml, respectively; and the regression equations were: Y = 3051758.7X-3210, r = 0.999 for chlorogenic acid, and Y = 2881690.6X-3820, r = 0.998 for isochlorogenic acid A.
Precision test
Five aliquots of 15 μL of test solutions were injected into the HPLC for determination of peak areas. The average peak area of chlorogenic acid and chlorogenic acid A were: 1260320 and 251691, respectively, and their RSDs were: 0.05% and 0.66%, indicating good precision.
Stability test
Test solutions under the "Precision test" were taken, and injected into the HPLC every 2, 4 ,6, 8, 10, 12, 18 and 24 h for determination of peak area, as well as stability within 24 h, the RSD of peak area was 0.22% for chlorogenic acid and 0.56% for isochlorogenic acid A, indicating that the test samples were stable within the investigated 24 h.
Reproducibility test
Nine aliquots of samples with the same batch number (140109) were taken in high, medium and low doses for extraction, preparation and determination as per the methods under the preparation of test samples and chromatographic conditions. Average mass fractions of the two types of chlorogenic acids upon determination of same batch of samples nine times were: 0.78 mg/g for chlorogenic acid, and 0.15 mg/g for isochlorogenic acid A; and RSDs were: 1.02% and 2.04%, respectively, indicating good reproducibility of the method.
Recovery test
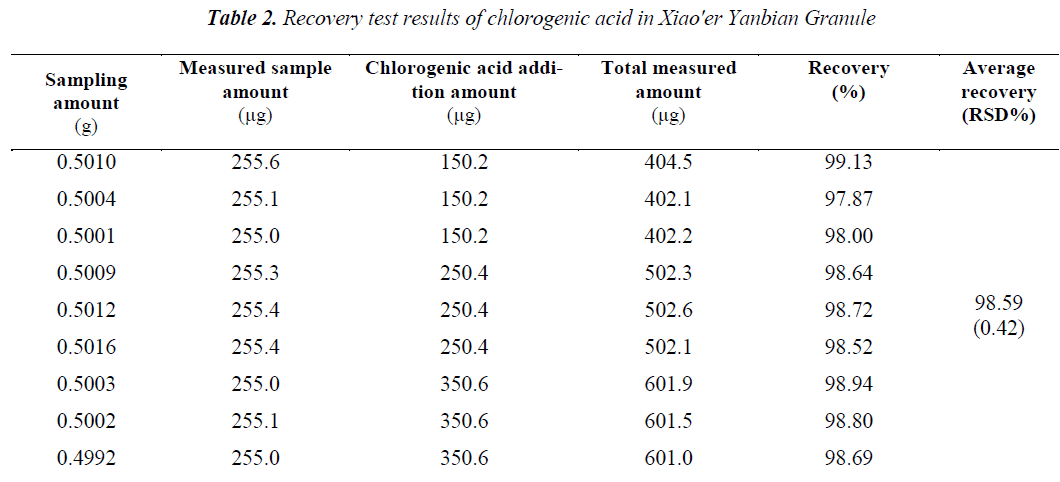
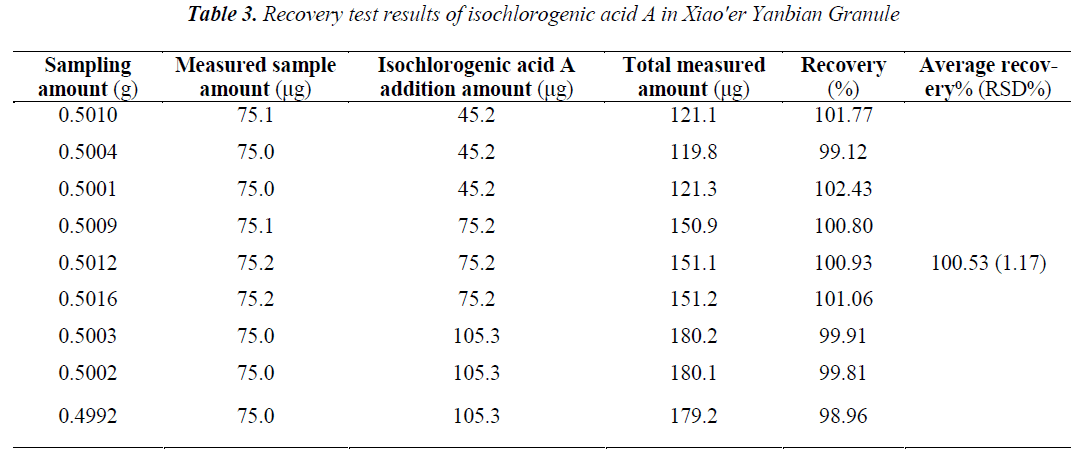
Sample recovery test was adopted. Nine aliquots of 0.5 g of same batch of samples (batch number: 140109) were taken in high, medium and low doses, plus a certain amount of reference substances, and extracted, prepared and determined as per the methods under the determination of test samples for calculation of recovery rates. Average recovery of the two types of chloro genic acids were 98.59% for chlorogenic acid, and 100.53% for isochlorogenic acid A, and RSDs (n = 9) were 0.42% and 1.17%, respectively. The results are shown in Tables 2,3.
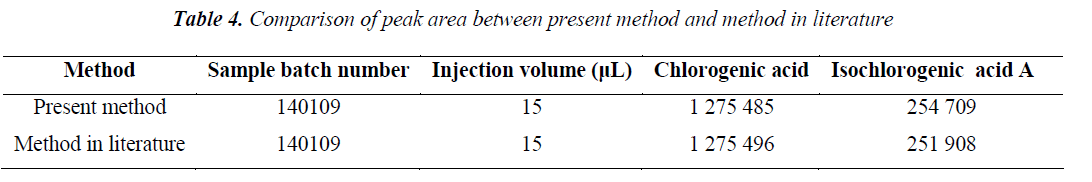
It can be seen from the data of gradient elution conditions reported in the literature in Tab. 6 that the isochlorogenic acid A contains the impurity peak, affecting the precision of the quantitative determination. Peak resolution chroma tograms showed that the resolution of present method was superior to the conditions reported in the literature, with the resolutions of both chlorogenic and isochlorogenic acids reaching 3 or above.
Comparison of present method with reported gradient determination method
Peak was determined by chromatographic methods used herein and reported in the literature [13], respectively, using the same column, the same sample solution and the same inject volume, the peak area data are shown in Tab. 4.
Discussion
Flos Lonicerae is one of the most important medicinal materials in Xiao'er Yanbian Granule. The major chemical constituents of the herb are also the major active ingredients of the granule. Flos Lonicerae ; which mainly contains phenolic acids dominated by chlorogenic acids, as well as anthocyanins has good antibacterial activity [6,10]. Therefore, to ensure the safety and efficacy for better quality control of Xiao'er Yanbian Granule, the determination of the chlorogenic acid content was done [16-17], i.e. the simultaneous determination of chlorogenic acid and isochlorogenic acid A contents in Xiao'er Yanbian Granule.
This study was the first to simultaneously determine the chlorogenic acid and ischlorogenic acid A contents in medicinal herb Flos Lonicerae and its preparation Xiao'er Yanbian Granule using the ordinary isocratic elution and reversed-phase column with acetonitrilemethanol- 0.1% phosphoric acid (20:6.5:73.5) as the mobile phase. By adjusting the proportion of the mobile phase, the quantitative chromatograms of the two constituents completed peak appearance in 20~30 min on three different columns, each peak had a good resolution, thus achieving a simple, fast, scientific, standardized and multi-index control of the quality of Xiao'er Yanbian Granule.
Compared with the reported gradient elution method, the present method reduces the performance requirements on instrument in terms of the contents of chlorogenic acid and chlorogenic acid A, where HPLC with gradient elution capability is no longer needed, and the quantitative determination of the two types of constituents is possible as long as there is an ordinary UV detector HPLC, thus substantially reducing the cost of experiments.
This method solves the problems of higher costs and cumbersome steps in testing of chlorogenic acid and isochlorogenic acid A in Xiao'er Yanbian Granule, thus achieving simple, fast and low-cost determination of drug quality. Meanwhile, it also provides the methodological basis and reference of multi-index determination for other Flos Lonicerae-containing preparations.
References
- WS3-B-1491-93, Drug Standards of the Ministry of Health - Traditional Chinese Medicinal Preparations.
- Yang HZ, Dong ZH, She J. Modern Study of Tradi- tional Chinese Medicine. Beijing: Academy Press 1998; 2938-2940.
- Zhang B, Yang RY, Zhao Y, Liu CZ. Separation of chlorogenic acid from honeysuckle crude extracts by macroporous resins. Journal of Chromatography B 2008; 867: 253-258.
- Chen L, Xin XL, Lan R, Yuan QP, Wang XJ, Li Y. Isolation of cyanidin 3-glucoside from blue honey-suckle fruits by high-speed counter-current chroma- tography. Food Chemistry 2014; 152: 386-390.
- Yuan Y, Yang J, Yu XD, Huang LQ, Lin SF. Antho- cyanins from buds of Lonicera japonica Thunb. var. chinensis (Wats.) Bak. Food Research International 2014; 62: 812-818.
- Peng LY, Mei SX, Jiang B, Zhou H, Sun HD. Con- stituents from Lonicera japonica Fitoterapia. Fi- toterapia 2000; 71: 713-715.
- Shi XW, Kong ZY. Quality control methods for Flos Lonicerae, Radix Scutellariae and their extract preparations: China, CN 1969953A 2007: 1632-1633.
- Xiang ZN, Ning ZX. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT - Food Sci- ence and Technology 2008; 41: 1189-1203.
- Zhao GL, Liu JJ, Lin D, Zhang XY, Wang H. Stud- ies on the chemical constituents in Flos Lonicerae. Chinese Traditional Patent Medicine 2002; 24: 973-976.
- HAN J, LV QY, JIN SY, ZHANG TT, JIN SX, LI XY, YUAN HL. Comparison of anti-bacterial activ- ity of three types of di-O-caffeoylquinic acids in Lo- nicera japonica flowers based on microcalorimetry. Chinese Journal of Natural Medicines 2014; 12: 108-113.
- Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia 1. Beijing: Chins Medical Science Press 2010: 448-449.
- China Food and Drug Administration, Technical Guidelines for Drug Research. Beijing: China Medi- cal Science Press 2005: 196-198.
- Chinese Pharmacopoeia Commission, Chinese Pharmacopoeia (Vol. 1). Beijing: China Medical Science Press 2010: 205-206.
- Zhou HY, Li D. Content Determination of Chloro- genic Acid in Qingkailing Injection by HPLC. China Pharmaceuticals 2011; 20: 36-37.
- Yu X. Determination of Chlorogenic Acid in Qiang- gan Oral Liquid by HPLC. China Pharmaceuticals 2011; 20: 41-42.
- Seo ON, Kim GS, Park S, Lee JH, Kim YH, Lee WS, Lee SJ, Kim CY, Jin JS, Choi SK, Shin SC. Deter- mination of polyphenol components of Lonicera ja- ponica Thunb. using liquid chromatography–tandem mass spectrometry: Contribution to the overall anti- oxidant activity. Food Chemistry 2012; 134: 572-577.
- Li J, Jin S, Zu YG, Luo M, Wang W, Zhao CJ, Fu YJ. Rapid preparative extraction and determination of major organic acids in honeysuckle (Lonicera japon- ica Thunb.) tea. Journal of Food Composition and Analysis 2014; 33: 139-145.
- Wang Z, Clifford MN, Sharp P. Analysis of chloro- genic acids in beverages prepared from Chinese health foods and investigation, in vitro, of effects on glucose absorption in cultured Caco-2 cells. Food Chemistry 2008; 108: 369-373.
- Wang TZ, Li YM, Wang ZX. RP-HPLC Quantitative Analysis of three kinds of organic acids in Flos Lo- nicerae. Chinese Journal of Pharmaceutical Analysis 2000; 20: 293-294.