Research Article - Journal of Dermatology Research and Skin Care (2023) Volume 7, Issue 3
Significant improvement of hair growth and appearance by Proteoglycan Replacement Therapy with Nourkrin® Woman - An investigator-initiated, treatment satisfaction study on women with diffuse hair loss from Mexico.
Jan Wadstein1*, Daniela A Guzman Sanchez2, Hugo Vicente Martinez Suarez3, Zaira Paulina Garcia Jimenez4
1Research and Development, Ostra Ronneholmsvagen Malmo, Sweden
2Clinica Dermatologica, Av. Pablo Neruda, Guadalajara, Jalisco, Mexico
3Marsu Dermatologia, Chulavista, Pue, México
4Fuera Calvicie Hair Restoration Clinic, Ciudad de Mexico, Mexico
- *Corresponding Author:
- Jan Wadstein
Research and Development
Ostra Ronneholmsvagen Malmo, Sweden
E-mail: dr.jan.wadstein@gmail.com
Received: 23-May-2023, Manuscript No. AADRSC-23-99655; Editor assigned: 26-May-2023, PreQC No. AADRSC-23-99655(PQ); Reviewed: 09-Jun-2023, QC No AADRSC-23-99655; Revised: 13-Jun-2023, Manuscript No. AADRSC-23-99655(R); Published: 21-Jun-2023, DOI:10.35841/aadrsc-7.3.147
Citation: Wadstein J, Sanchez D A G , Suarez H V M, Jimenez Z P G. Significant improvement of hair growth and appearance by Proteoglycan Replacement Therapy with Nourkrin® Woman - An investigator-initiated, treatment satisfaction study on women with diffuse hair loss from Mexico. Dermatol Res Skin Care. 2023; 7(3):147
Abstract
Background: Proteoglycan Replacement Therapy (PRT) is an emerging anti hair loss treatment using a proprietary complex of bioactive proteoglycans (Marilex®) to stimulate and maintain anagen in hair follicles affected by Female Pattern Hair Loss (FPHL) and Telogen Effluvium (TE). NourkrinA® woman with Marilex® (Pharma Medico Aps) works by preventing/treating proteoglycan dysmetabolism and Proteoglycan Follicular Atrophy (PFA) through augmenting the expression of follicular proteoglycans. Our objective was to evaluate the patient-reported efficacy and satisfaction from PRT with NourkrinA®. Methods: This was an observational treatment satisfaction study on 86 women (mean age=41.20 years) from Mexico with diffuse hair loss due to FPHL or TE, who voluntarily started a 6-month course of PRT with NourkrinA® woman (providing 600 mg Marilex®/day). Treatment efficacy and tolerability were assessed at mid-study and endpoint using structured, self-administered questionnaires. All phases of this study were conducted under the supervision of the World Hair Council.
Results: At baseline, 61% of volunteers had moderate-to-severe diffuse hair loss, while only 10% had a history of prior treatment. Hair growth was enhanced in 95% and 89% of patients after three and six months respectively, with prominent changes in >78%. NourkrinA® also improved hair quality/appearance in 97% of users after three months and 88% at endpoint. Importantly, this treatment raised the level of self-confidence in 8/10 of patients. Overall, treatment satisfaction rate in this cohort was 94% at month three and 89% at month six. No clinically significant side effects were reported during the course of the study.
Conclusions: This research suggests that NourkrinA® woman with Marilex® has positive effects on hair growth and appearance in women with FPHL and TE, which begin to emerge after three months of treatment. Treating hair loss with NourkrinA® woman can also improve self-confidence in the majority of patients. Overall, the level of treatment satisfaction was exceptionally high in this study at both time points.
Keywords
Female pattern hair loss, Proteoglycans, Proteoglycan replacement therapy, Nourkrin, Marilex, Patient outcome assessment, Treatment satisfaction.
Introduction
Proteoglycan Replacement Therapy (PRT) is now a wellrecognised approach to diffuse hair loss in women, which is widely utilised by dermatologists and trichologists around the globe. In this approach, a specific treatment containing a marine-derived extract, Nourkrin® Woman with Marilex® (Pharma Medico ApS, Aarhus, Denmark), is administered orally for a minimum of 6-12 months to women with Female Pattern Hair Loss (FPHL) or Telogen Effluvium (TE). The idea of oral prescription of specific natural proteoglycans for the treatment of hair loss was the brainchild of Jan Wadstein and Erling Thom introduced in late 1970s. These researchers noticed the importance of the biological activity of proteoglycans for healthy hair growth and cycling and hypothesised that enhancing their expression by treatment can support hair growth and prevent immature hair loss as described in a recently published review paper [1]. This targeted treatment was later developed in collaboration with Pharma Medico ApS using a sophisticated processing method to extract bioactive proteoglycans from marine animals; and thereby, Marilex® was born.
Marilex®, the main active ingredient in Nourkrin® Woman, contains a concentrated combination of hair-specific proteoglycans, e.g., versican, decorin, and syndecan. These bioactive macromolecules are absorbed by endocytosis from the small intestine and target the replicating parts of hair follicles [2]. The clinical efficacy of PRT with Nourkrin® is confirmed by several quantitative and qualitative trials on hair loss patients of diverse national and racial backgrounds. In two randomised, placebo-controlled, clinical trials, a total of 115 patients with diffuse hair loss were treated with Nourkrin® with Marilex® for 6-12 months. Just after six months, hair count per unit area was significantly increased by ~35%, which was further improved by continuing the treatment for a total of 12 months. Subjects treated by Nourkrin® expressed a constiderably higher treatment satisfaction compared to the ones who received placebo [3,4]. Research also revealed that the clinical effectiveness of PRT with Nourkrin® can lead to meaningful improvements in patients’ quality of life [5].
‘Patient satisfaction’ is a healthcare index of great importance in clinical medicine, particularly in assessing dermatological and trichological treatments. Satisfaction can be defined as the extent of an individual’s experience compared with his or her expectations [6]. Patients’ satisfaction from a treatment is often assessed via specially designed questionnaires. Despite their importance, patient satisfaction surveys often receive little attention from clinicians and clinical researchers and are neglected in standard clinical evaluation of novel treatments. In the case of Nourkrin®, this common shortcoming is avoided, and a series of subjective clinical studies are being or had been conducted to draw a complete picture of patients’ satisfaction level with PRT. It is common knowledge that patient satisfaction does not merely depend on the objective efficacy of a treatment but is also influenced by cultural and racial factors [7]. Therefore, patient satisfaction surveys need to be replicated in different countries with diverse racial/ cultural backgrounds. The results of such surveys from the United Kingdom and Brazil have already been published [8,9]. In the present paper, we report our findings from a subjective, clinical study on women with diffuse hair loss from Mexico.
Materials and Methods
Study design
This was an observational, patient-reported, treatment satisfaction cohort with a concurrent, 6-month follow-up period. In this study, individuals who voluntarily chose to start a 6-month course of monotherapy with Nourkrin® Woman (Pharma Medico Aps, Aarhus, Denmark) for treating their hair loss condition were followed and examined. Nourkrin® Woman is a per oral treatment administered as 2 tablets per day delivering 600 mg of Marilex®. This active complex is a proprietary natural extract rich in hair growth-stimulating proteoglycans such as versican and decorin.
Our co-primary outcomes were the change in self-reported hair growth, hair appearance/quality, and self-confidence plus their overall satisfaction rate with the treatment. Our secondary outcome was the safety/tolerability of PRT with Nourkrin® Woman. All study outcomes were assessed three and six months after the start of the treatment using a structured, selfadministered questionnaire, which was translated to Spanish. The study questionnaire (available in the Supplementary Material) consisted of 2- and 4-point (Likert-type scale) questions as well as queries on newly-onset symptoms or potential side effects. The participants had the possibility to contact a clinician through a hotline for reporting side effects and making urgent inquiries in connection with the treatment.
Study participants
Volunteers who met the following eligibility criteria were enrolled into the study: 1- females of 18-64 years of age; 2- having a physician-made diagnosis of FPHL or TE; 3- not being under treatment for hair loss at or three months prior to the time of enrolment. The initial screening phase was carried out by a number of collaborating dermatologists practicing in outpatient clinics in Mexico. 124 women with a chief complain of diffuse hair loss were screened from whom 86 eligible individuals were selected for enrolment into the study. The stage of hair loss at baseline was assessed using Ludwig classification scale for FPHL [10].
Prior to enrolment, comprehensible information on the design and aims of the study was provided to the participants in addition to their rights and responsibilities. Subsequently, a written consent was obtained. Study investigators have instructed the patients against using any form of anti-hair loss medications or supplements, undergoing laser treatment, hair transplantation or other elective surgical procedures involving the scalp throughout the follow-up period. Additionally, taking medications known to affect hair growth (e.g., contraceptive pills, anabolic steroids, immunomodulators and cytotoxic or cytostatic drugs) was not allowed. The participants also agreed to maintain their usual hairstyling practices for the duration of the study. Pregnant and breastfeeding women as well as the individuals with a serious underlying disorder or known allergy to fish or shellfish were not enrolled.
In this study, all stages of designing the study protocol, recruitment of subjects, and evaluation of outcomes were approved and supervised by the expert members of the World Hair Council (WHC). WHC operates as a non-profit organization of hair loss experts including dermatologists, trichologists, and aesthetic professionals (https://worldhaircouncil.com).
Results
All 86 enrolled women attended the midpoint evaluation; whereas three participants failed to submit their questionnaires due to personal reasons at the end of the study and were eliminated from the final assessment. Data from these lost-tofollow- up subjects is included in the midpoint results.
Baseline characteristics of the study population is summarised in the Table. As shown, volunteers were young to middleaged women with mild to severe degrees of diffuse hair loss. Surprisingly, around 90% of our patients have not received effective medical treatment before joining the current study despite their significant hair loss problem.
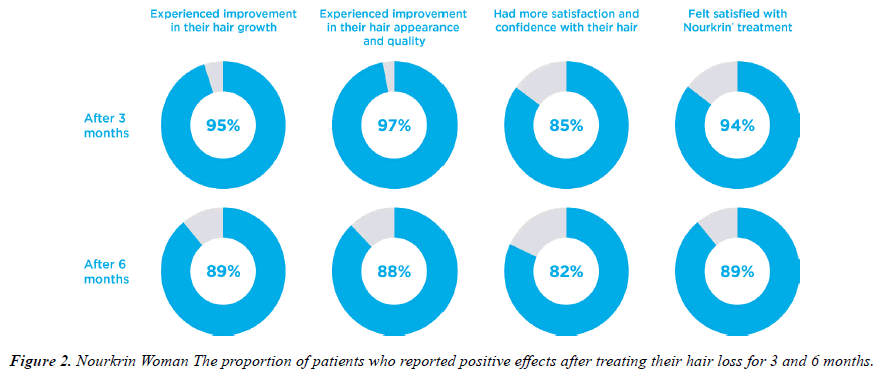
The 3-6 months study questionnaires contained one question associated with each of the main study outcomes. Figure 1 depicts the descriptive statistics of subjects’ responses to the efficacy question. As shown, 95% of patients started noticing positive effects of Nourkrin® therapy on the growth of their hair after three months, and the majority (97%) reported simultaneous improvements in the appearance of their hair. As a result, 97% of users were satisfied with PRT with Nourkrin® and were willing to continue the treatment after the first three months. At the end of the follow-up period, 74 out of 83 women with diffuse hair loss (89%) observed a visible increase in the growth of their hair, and 73 subjects (88%) reported having better hair appearance and quality (Figure 1). Overall, the treatment satisfaction rate in this cohort was 94% at month three and 89% at month six.
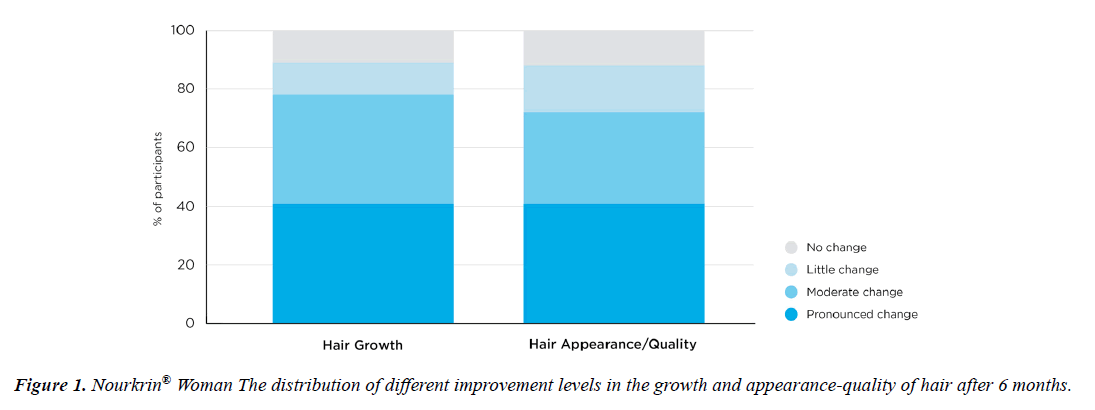
A Likert-type scaling of the changes in patients’ hair growth and appearance at endpoint is presented in Figure 2. According to the Figure, Nourkrin® therapy induced moderateto- pronounced changes in hair growth and appearance in more than 78% and 72% of the treated participants respectively.
An important finding of this study was the significant effect of Nourkrin® on hair satisfaction and self-confidence. More than 80% of treated women stated that consuming Nourkrin® significantly helped them to feel more confident with the appearance of their hair (see Figure 1), which raised their level of self-confidence in social interactions.
Patients were also closely monitored during the course of the study to detect any newly-onset symptoms or side effects. However, no cases of clinically significant side effects were reported by the participants that can be attributed to PRT with Nourkrin® Woman , and no withdrawals due to tolerability issues occurred.
Discussion
Patient-reported, treatment satisfaction studies are beneficial tools, which provide a realistic insight into the patient’s perspective over a particular treatment or service. This research method is thus widely used in different fields of clinical medicine [11,12]. The level of patient satisfaction with a particular healthcare intervention depends on a variety of factors, including the interaction of one’s expectations and preferences with the perceived efficacy of the intervention. Achieving a high level of patient satisfaction is particularly important in the practice of cosmetic dermatology and trichology due to the strong psychological and emotional impact of hair and skin disorders.
A targeted subjective research method was utilised in this study to investigate the perceived clinical efficacy of PRT with Nourkrin® Woman in females from Mexico. Similar studies have previously been conducted in other populations from the UK and Brazil, which findings are published in peerreviewed journals. Similar to our observations, more than 90% of hair loss patients with either a European or South American background experienced visible improvements in the growth and quality of their hair after treatment with Nourkrin® Woman. This conformity indicates that the perceived efficacy of Nourkrin® Woman with Marilex® is independent of patients’ racial/national background. The overall treatment satisfaction rate also exceeded 90% in all the studied populations, which roots probably in a high clinical efficacy together with the convenient dosing and administration route of PRT. Further research is, however, required in other parts of the world, e.g., Asian, and Middle Eastern countries, in order to confirm this conclusion.
Marilex®, the main active ingredient of Nourkrin® Woman , is a proprietary mixture of marine-derived proteoglycans. The action mechanisms via which Marilex® can improve the function of hair follicles have been a subject of decadeslong scientific research. The aggregation of evidence derived from this research endeavour is outlined in two literature reviews by Wadstein, et al. [1] and Thom, et al. [13]. These authors elaborate that the bioactive proteoglycans in Marilex® induce and maintain anagenic hair growth via promoting the survival and proliferation of key follicular cell populations. Certain marine-derived proteoglycans, such as versican and perlecan, have growth factor-like activities, which promote the proliferation and migration of fibroblasts and dermal papilla cells [14,15]. Moreover, the anti-inflammatory and antioxidative properties of proteoglycans protect the actively growing cells from oxidative damage and apoptosis. The ability of proteoglycan treatment in triggering hair growth in dormant follicles and blocking premature catagen initiation is directly shown in-vivo [16]. Hence, Marilex® works by inducing anagen in dormant and telogenic follicles and supporting the growth of anagenic follicles. This effect, known as the ‘hair growth cycle normalising’ effect, was experienced by the majority of our patients throughout the course of the study.
Nourkrin® Woman users also noticed clear improvements in the quality and appearance of their hair. This finding can be attributed to the hair thickening and anti-miniaturisation effects of PRT. In patients with pattern hair loss, the anabolic/ catabolic balance of proteoglycans is disturbed leading to a state of insufficient proteoglycan expression in the follicle, known as Follicular Hypoglycania (FHG). Untreated FHG can progress towards a gradual shrinkage of the hair miniorgan due to Proteoglycan Follicular Atrophy (PFA), presented clinically as follicular miniaturisation. PRT can delay or reverse the development of PFA by boosting the concentration of key proteoglycans in the follicle. This unique effect is referred to as the ‘anti-miniaturisation effect’ of PRT. In addition, certain proteoglycans also possess a number of anti-inflammatory properties, which can greatly contribute to the treatment of hair loss, particularly in patients with FPHL.
Parallel with evaluating the visible, physical changes, it is also important to explore the impact of treatment on psychological wellbeing of hair loss patients. Clinical research since the 1990s corroborated the fact that hair loss leaves more serious and pervasive psychosocial impacts on women compared to men. About twice as many women with PHL as men express that they are ‘very’ to ‘extremely’ upset by their hair loss [17]. This is fuelled by the cultural association of hair with gender identity, sexuality, and attractiveness in women; thus, any hair imperfection is perceived as deeply stressful and damaging to a woman’s body image and self-confidence. This psychological damage is to such an extent that more than one fourth of women with hair loss express the symptoms of body dysmorphic disorder [18]. Naturally, addressing hair loss, as the original aetiology of this complication, can be the first-line therapeutic approach. In this study, PRT with Nourkrin® succeeded in improving the confidence of >80% of patients in their hair by promoting its fullness/ appearance. This effect, in addition to its positive mental influences, can also contribute to the normalisation of hair growth cycle through mitigating psychological stress, which is a potent catagen inducer [19].
Nourkrin® displayed good tolerability and desirable safety profile in our study. In principle, the medications used for the treatment of non-life threatening, cosmetic disorders need to be safe with low likelihood of causing serious side effects. Accordingly, inflicting such side effects as fetal malformation, hypotension, hypertrichosis, and serious psychiatric complications, which can happen with certain conventional hair loss medications, is not medically justified. In light of this, a significant clinical advantage of Nourkrin® is its tolerability and benign side effect prolife, i.e., important side effects was identified neither in the previous clinical trials and observational studies, nor during the present cohort.
Conclusion
We investigated the patient-reported, clinical efficacy and treatment satisfaction rate of PRT with Nourkrin® Woman with Marilex® on Mexican women with diffuse hair loss. The positive effects of treatment appeared after three months in the absolute majority of patients and persisted until the end of the study. 9 out of 10 participants also experienced simultaneous improvements in the quality of their hair. This research provides evidence that treating hair loss with Nourkrin® can provide psychological benefits and improve the self-confidence of patients. Similar to previous studies, most patients were completely satisfied with treating their hair loss issue with Nourkrin® at both mid-study and endpoint. In conclusion, our findings suggest that oral PRT with Nourkrin® is an effective treatment for diffuse hair loss caused by FPHL and TE with an optimal tolerability and safety profile.
Conflicts of interest
The authors report no conflicts of interest that may affect the integrity of this work.
References
- Wadstein J, Thom E, Gadzhigoroeva A. Integral roles of specific proteoglycans in hair growth and hair loss: mechanisms behind the bioactivity of proteoglycan replacement therapy with Nourkrin® with Marilex® in pattern hair loss and telogen effluvium. Dermatol Res Pract. 2020;2020.
- Tsuchiya Y, Tomita M, Tsuboi M, et al. Absorption of proteoglycan via clathrin-mediated endocytosis in the small intestine of rats. Biosci Biotech Biochem. 2013;77(3):654-6.
- Thom E. Efficacy and tolerability of Hairgain® in individuals with hair loss: a placebo-controlled, double-blind study. J Int Med Res. 2001;29(1):2-6.
- Thom E. Nourkrin®: Objective and subjective effects and tolerability in persons with hair loss. J Int Med Res. 2006;34(5):514-9.
- Kingsley DH, Thom E. Cosmetic hair treatments improve quality of life in women with female pattern hair loss. J Appl Cosmetol. 2012;30(2):49-59.
- Biering P, Jensen VH. The concept of patient satisfaction in adolescent psychiatric care: A qualitative study. J Child Adolesc Psychiatr Nurs. 2010;23(3):143-50.
- Liao L, Chung S, Altamirano J, et al. The association between Asian patient race/ethnicity and lower satisfaction scores. BMC Health Serv Res. 2020;20(1):1-1.
- Thom E, Wadstein J. Treating Female Diffuse Hair Loss using Nourkrin® Woman (with Marilex®)-An Open-label, Subjective, Outcome Study on Hair Growth and Appearance, Self-Confidence and Treatment Satisfaction. J Clin Dermatol Ther. 2019;5:037.
- Mattos Simoes M, Thom E, Wadstein JN. Woman with Marilex® enhances hair growth and appearance and improves hair confidence in women with diffuse hair loss from Brazil: An investigator-initiated clinical study. J Clin Investigat Dermatol. 2020;8(1):4.
- Ludwig E. Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. 1977;97(3):247-54.
- Revicki D. Patient assessment of treatment satisfaction: Methods and practical issues. Gut. 2004;53(suppl 4):iv40-4.
- Bamber L, Wang MY, Prins MH, et al. Patient-reported treatment satisfaction with oral rivaroxaban versus standard therapy in the treatment of acute symptomatic deep-vein thrombosis. Thromb Haemost. 2013;110(10):732-41.
- Thom E, Wadstein J, Thom EW, et al. Treatment of hair thinning and hair ageing with specific lectican and leucine proteoglycans. A review. J Appl Cosmetol. 2014;32(3/4):106-15.
- Yang Y, Li Y, Wang Y, et al. Versican gene: Regulation by the ß-catenin signaling pathway plays a significant role in dermal papilla cell aggregative growth. J Dermatol Sci. 2012;68(3):157-63.
- Theocharis AD, Skandalis SS, Tzanakakis GN, et al. Proteoglycans in health and disease: Novel roles for proteoglycans in malignancy and their pharmacological targeting. The FEBS journal. 2010;277(19):3904-23.
- Jing J, Wu XJ, Li YL, et al. Expression of decorin throughout the murine hair follicle cycle: hair cycle dependence and anagen phase prolongation. Exper Dermatol. 2014;23(7):486-91.
- Cash TF, Price VH, Savin RC. Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. 1993;29(4):568-75.
- Dogruk Kacar S, Ozuguz P, Bagcioglu E, et al. Frequency of body dysmorphic disorder among patients with complaints of hair loss. Int J Dermatol. 2016;55(4):425-9.
- Arck PC, Handjiski B, Peters EM, et al. Stress inhibits hair growth in mice by induction of premature catagen development and deleterious perifollicular inflammatory events via neuropeptide substance P-dependent pathways. Am J Pathol. 2003;162(3):803-14.
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref
Indexed at, Google Scholar, Cross Ref