Research Article - Biomedical Research (2017) Volume 28, Issue 13
Significance of microRNA-29a as a biological marker in evaluating the effect of mycophenolate mofetil treatment of IgA nephropathy
Zhang Qianqian1,2, Hou Huijuan3, Fu Xueguo3, Wei Jianxin2, Zhou Shuhua2 and Wang Rong1*
1Department of Urology, Shandong Provincial Hospital affiliated To Shandong University, Jinan, Shandong, PR China
2Department of Urology, Dezhou People’s Hospital, Dezhou, Shandong, PR China
3Department of Cardiology, Dezhou People’s Hospital, Dezhou, Shandong, China
- *Corresponding Author:
- Wang Rong
Department of Urology Shandong
Provincial Hospital affiliated To Shandong University, PR China
Accepted on May 18, 2017
Abstract
Objective: This study was designed to analyse the urinary, serum and renal expression of miR-29a of patients diagnosed with IgAN, aiming to elucidate the effect of MMF upon the expression level of miR-29a in IgAN patients.
Methods: Thirty-three patients diagnosed with IgAN by biopsy were assigned in the IgAN group. Serum and urine sampling was collected from 15 healthy volunteers as healthy control group, normal renal tissue from the nephrectomy specimen of 15 patients with renal cell carcinoma in the control group. RTPCR was performed to compare miR-29a level between three groups and explore the effect of MMF on expression level of urinary and serum miR-29a.
Results: Compared with control group, the urinary, serum and renal expression of miR-29a in IgAN group were decreased (P<0.05). Urinary and serum expression of miR-29a inversely correlated with proteinuria (r=-0.322, P<0.05; r=-0.356, P<0.05), and glomerulosclerosis (r=-0.427, P<0.05; r=-0.517, P<0.05) in IgAN group. Urinary expression of miR-29a positively correlated with evaluated Glomerular Filtration Rate (eGFR) (r=0.328, P<0.05), inversely correlated with diastolic blood pressure (r=-0.421, P<0.05) in the IgAN group. Serum and intra-renal expression of miR-29a was positively correlated with urinary expression of miR-29a (r=0.713, P<0.05; r=0.322, P<0.05). The serum and urinary levels of miR-29a in the MMF group were significantly higher than those in the IgAN group (both P<0.05).
Conclusion: Urinary and serum miR-29a level were closely correlated with the progression of IgAN. MMF could up-regulate the serum and urinary levels of miR-29a in IgAN patients. Serum and urinary miR-29a probably serve as biological markers to reflect the effect of MMF treated with IgAN.
Keywords
MicroRNA-29a, Immunoglobulin A nephropathy, Mycophenolate mofetil, Kidney fibrosis.
Introduction
IgA nephropathy (IgAN) is regarded as a common glomerulonephritis with a prevalence of >50 % of primary glomerulonephritis diagnosed by pathological biopsy [1]. Approximate 15-40% of IgAN patients are likely to progress to the End-Stage Renal Disease (ESRD) [2,3]. At present, renal function and proteinuria are regarded as useful prognostic markers of IgAN. Nevertheless, these markers cannot precisely predict outcomes due to the heterogeneity of the disease [4]. Proliferation of mesangial cells with expansion of extracellular matrix in light microscopy, mesangial deposition of IgA in the immunofluorescence and paramesangial immune deposits in electron microscopy represent the histological features of IgAN. Assessment of the degree of glomerular and tubulointerstitial can be used to predict renal outcomes [5]. Renal biopsy has potential complications, and repeated renal biopsy is technically difficult. Exploring appropriate biomarkers which reflect disease severity and progression are urgently required in the management of IgAN patients [6].
Following the initial mesangial deposition of IgA, there is progressive glomerulosclerosis and tubulointerstitial fibrosis in IgAN [7]. Renal fibrosis plays an important role in IgAN progression to chronic renal failure, which are common pathological features of Chronic Kidney Disease (CKD) progress to ESRD. Available evidence shows that Transforming Growth Factor-β1 (TGF-β1) is the major mediator of progressive renal fibrosis in IgAN [8-10], which is largely via the SMAD-dependent pathway [11,12]. However, the molecular mechanisms underlying the link between TGF- β1/SMAD signaling pathway and disease progression in IgAN remain unknown.
MicroRNAs (miRNAs) are noncoding, single-stranded RNA molecules of approximately 21 to 23 nucleotides in length, it is involved primarily in the control of gene expression at post transcriptional level and plays important roles in many physiological and pathological processes [13-15]. miRNAs participate in each ring of cellular process, and dys-regulation of miRNA has been correlated with multiple human diseases including CKD [16,17]. Previous studies suggest that TGF-β1/ Smad3 signaling promotes renal fibrosis by inhibiting miR-29 [18]. In mouse models, the expression level of miR-29 is downregulated along with the progression of kidney fibrosis in obstructive nephropathy. Instead, mice with knockout of Smad3 presented with the up-regulated expression of miR-29 over the lack of kidney fibrosis in obstruction mouse models [19].
Mycophenolate Mofetil (MMF) could significant reduce proteinuria, and improve renal function with patients of IgAN. In streptozotocin-induced diabetic nephropathy, the administration of MMF prevented the development of proteinuria, macrophage infiltration and glomerulosclerosis [20] and resulted in reduced expression level of Intercellular Adhesion Molecule-1 (ICAM-1), TGF-β1 and Monocyte Chemoattractant Protein-1 (MCP-1) [21,22].
Urinary level of miR-29a was decreased in IgAN patients [23]. However, serum, urinary and renal levels of miR-29a of patients with IgAN remain elusive. MMF has been applied in the treatment of IgAN and indicates that MMF can up-regulate the miR-29a expression and inhibit renal fibrosis in patients diagnosed with IgAN. In the present study, real-time PCR was performed to explore serum, urinary and intra-renal expression of miR-29a and its function in renal fibrosis of patients with IgAN, aiming to elucidate whether the changes of miR-29a expression are the mechanism underlying clinical efficacy of MMF in the management of IgAN.
Materials and Methods
Baseline data
Thirty-three consecutive patients with IgAN confirmed by kidney biopsy between March 2014 and November 2015 in Shandong Provincial Hospital affiliated To Shandong University were assigned in the IgAN group, and 47 patients with IgAN treated with MMF for 3 months were allocated into the MMF group. Serum and urine from 15 healthy volunteers as healthy control group, normal renal nephrectomy specimen from 15 patients with kidney cell cancer as control group. Patients complicated with alternative renal diseases were excluded. Study procedures were approved by the Ethics Committee of Shandong Provincial Hospital affiliated To Shandong University. Informed consents were obtained from all patients. Clinical and pathological data including serum creatinine, 24 h urine protein, hemoglobin, albumin, blood pressure, glomerulosclerosis, and crescent were recorded. Evaluated Glomerular Filtration Rate (eGFR) was estimated by the standard equation [24].
Patients in MMF group were treated with MMF combined with low-dose corticosteroids and Angiotensin Receptor Antagonist (ARB), MMF starting dose as 30 mg/kg/d, 1.5-2.0 g/d, orally twice, appropriate reduction after 6 months of maintenance therapy, total treatment course for 1.5 y; initial dose of glucocorticoid prednisone was 0.5 mg/kg/d, appropriate reduction after six months of maintenance therapy, total treatment course for 1.5 y. All IgAN patients were treated with conventional dose of 100 mg/d losartan.
Sampling preparation
The urine sample and 5 ml of whole blood was prepared by each patient for urinary and serum miR-29a expression study. Blood and urine samples were kept at 4°C for 5 h. Subsequently, these obtained samples were centrifuged at 3000 g at 4°C for 30 min. The urine and serum supernatants were centrifuged at 12,000 g at 4°C for 15 min and kept at 80°C for subsequent experiment.
The kidney tissues were placed in 10% of neutral-buffered formaldehyde immediately after renal biopsy, and subsequently then soaked in the alcohol and embedded in paraffin for staining to analyse the expression level of renal miRNA. Five 10-mm formalin-fixed sections were obtained and handled using a microtome. These sections were soaked in xylene at 50°C for 3 min and rinsed twice with the absolute ethanol. Subsequently, the pellet was dried at room temperature for 30 min, and used for intra-renal miR-29a expression.
RNA extraction
Serum and urine RNA sampling was extracted by mirVana PARIS kit (Ambion) according to the manufacturer’s instructions. In brief, 500 μl serum was mixed with an equivalent quantity of 2X denaturing solution, and total RNA was eluted. Total RNA derived from the obtained cells was isolated by the RNAiso Plus (Takara, Japan) according to the manufacturer’s instructions.
Detection of miR-29a level
For miR-29a, 5.5 μl Tailed RNA was mixed with 2.5 μl 4X reaction buffer, 1.0 μl PolyA/RT enzyme, 0.5 μl miRNA RT primer (1 μM), 0.5 μl Internal control RT primer (1 μM) and made up to 10 μl with H2O at 42°C for 60 min, 75°C for 10 min, diluted for 4-15 times, added with 30-140 μl water. Urinary, serum and intra-renal miR-29a were quantified by RTQPCR. For RT-QPCR, 8 μl Diluted cDNA (5 times dilution) was mixed with 2 μl 10X self Taq buffer, 2 μl 2 mM dNTP mix, 0.5 μl Self Taq Polymerase, 0.4 μl 10 μM Forward primer, 0.4 μl 10 μM Reverse primer, 0.5 μl 10 μM Probe, 0.2 μl ROX reference dye (100X, Sigma) and 6.0 μl H2O were mixed to obtain 20 μl reaction volume. Each sample was run in triplicate. RT-qPCR was performed at 95°C for 30 s for 40 cycles at 95°C for 10 s and 60°C for 30 s. RNU48 (Applied Biosystems) was used as house-keeping genes to normalize the miRNA expression [25]. To investigate the expression for all targets of varying sampling, the 2-ΔCt method was utilized for quantitation. ΔCT=AVG(miR-29a CT)-AVG (house-keeping genes CT).
Pathological examination
The severity of kidney fibrosis was assessed by Periodic Acid Schiff staining (PAS). The severity of renal pathological damage was scored by experienced pathologists who were blinded to the results of this experiment. The severity of glomerulosclerosis and crescent was represented by the proportion of sclerotic glomeruli and crescent in all glomeruli.
Statistical analysis
SPSS 19.0 statistical software was adopted for data analysis (SPSS Inc., Chicago, IL). All data were expressed in mean ± standard deviation. T-test was used for comparison between the two groups for data normally distributed, and χ2 test for the others. Mann-Whitney U test was used to compare the expression levels between groups and Spearman’s correlation to analyse the relationship between gene expression and clinical indexes. A P value of less than 0.05 was considered statistical significance.
Results
Baseline data
Demographic and baseline clinical and pathological data of the study subjects were illustrated in Table 1. The difference of clinical and pathological data between IgAN and MMF group was not statistically significant, which matched the results between two groups (all P>0.05).
| IgAN | MMF | T/ χ2 | P value | |||
|---|---|---|---|---|---|---|
| No. of case | 33 | 47 | ||||
| Gender/male/female | 16:17 | 20:27 | 0.276 | 0.6 | ||
| Age/year | 35.55 ± 9.66 | 36.02 ± 10.53 | -0.206 | 0.837 | ||
| SBP/mmHg | 136.52 ± 20.06 | 140.28 ± 17.44 | -0.892 | 0.375 | ||
| DBP/mmHg | 85.97 ± 15.15 | 87.70 ± 11.88 | -0.573 | 0.569 | ||
| Proteinuria/g/day | 1.92 ± 1.75 | 2.10 ± 1.57 | -0.494 | 0.623 | ||
| Hemoglobin/g/L | 129.34 ± 19.46 | 128.31 ± 18.51 | 0.239 | 0.812 | ||
| Albumin/g/L | 36.10 ± 5.45 37 | 56 ± 5.72 | -1.147 | 0.255 | ||
| Serum creatinine/µmol/L | 149.65 ± 74.62 | 138.48 ± 75.02 | 0.657 | 0.513 | ||
| eGFR/ml/min/1.73m2 | 59.61 ± 34.83 | 67.09 ± 43.99 | -0.813 | 0.418 |
Abbreviation: IgAN: Immunoglobulin A Nephropathy; eGFR: Evaluated Glomerular Filtration Rate; SBP: Systolic Blood Pressure; DBP: Diastolic Blood Pressure.
Table 1. Demographic, clinical and pathological data of the subjects.
Level of urinary, serum and intra-renal miR-29a
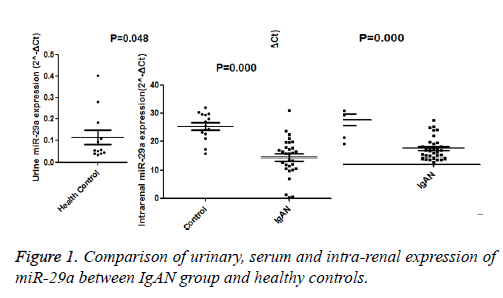
The urine, renal and serum expression of miR-29a were compared and summarized in Figure 1. Urinary expression of miR-29a of IgAN patients was significantly lower than those in the control group (miR-29a (A): 0.047 (0.027-0.071) versus 0.052 (0.038-0.162), P<0.05); serum expression of miR-29a of patients with IgAN was also significantly down-regulated than that in the control group (miR-29a (B): 0.355 (0.170-0.546) versus 1.254 (0.687-1.491), P<0.05); intra-renal expression of miR-29a of patients with IgAN was significantly lower than that of controls (miR-29a (C): 15.608 (10.458-18.110) versus 25.304 (22.491–29.532), P<0.05).
MiR-29a level and clinical, pathological data
Since serum, urinary and renal expression of miR-29a significantly differed between the study and control groups, the study analysed the relationship between miR-29a level and clinopathological parameters. The urinary expression of miR-29a was significantly correlated with proteinuria (r=-0.336, P<0.05), eGFR (r=0.358, P=0.023), glomerulosclerosis (r=-0.537, P<0.05) and DBP (r=-0.471, P<0.05). Similarly, serum expression of miR-29a significantly correlated with proteinuria (r=-0.365, P<0.05) and glomerulosclerosis (r=-0.487, P<0.05). There was no significant correlation between intra-renal expression of miR-29a and clinical and pathological data.
Relationship among urinary, serum and intra-renal expression of miR-29a
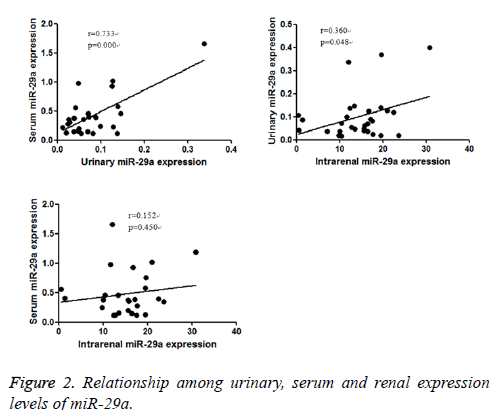
Since urinary, serum and intra-renal expression of miR-29a of patients with IgAN were decreased consistently, the study further to explore the relationship of urinary, serum and intrarenal miR-29a level in IgAN group. It found that urinary expression of miR-29a positively correlated with serum expression of miR-29a (r=0.733, P<0.05), and intra-renal expression of miR-29a (r=0.360, P<0.05).There was no significant relationship between serum and intra-renal expression levels of miR-29a (r=0.152, P <0.05) (Figure 2).
Effect of MMF treatment on serum and urinary expression of miR-29a
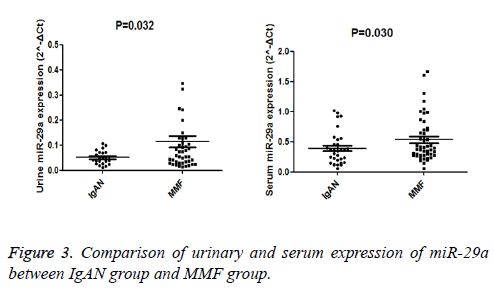
Since urinary, serum and intra-renal expression of miR-29a of patients with IgAN was decreased, It also found the serum and urinary level of miR-29a in MMF group was significantly higher than IgAN group (P=0.032, P<0.05), as illustrated in Figure 3.
Discussion
IgAN is frequently encountered in Asian countries. Poor clinical prognosis is obtained in the patients diagnosed with massive hematuria, renal dysfunction and histological lesions, etc. [26]. The degree of renal fibrosis is closely related to prognosis of IgAN. Although many studies investigated urinary protein and mRNA as potential biological markers for kidney diseases, the expression of miRNA in serum with IgAN has been rarely investigated. However, urinary and serum miRNAs have several theoretical advantages to be utilized as biological markers for renal illness. First, global miRNA quantification approach is more efficient compared with the detection of conventional protein markers [27]. Secondly, miRNAs are more stable and abundant compared with traditional mRNAs [28,29]. Third, a single miRNA species is able mediate the expression of different genes [30]. This study aimed to analyse the serum, urinary and renal expression of miR-29a of IgAN patients, and investigate whether miR-29a expression is associated with the clinical efficacy of MMF in the treatment of IgAN.
MiR-29a is one of the miR-29 family members, including miR-29a, miR-29b, and miR-29c. Previous studies suggested that miR-29 family was closely related renal fibrosis mediated by TGF-β1/Smad3 signaling pathway [19].
In the present study, it examined the expression level of miR-29a in urine, serum and kidney biopsy of patients with IgAN and found that urinary, serum and renal expression of miR-29a of IgAN patients was significantly lower compared with those in the control group. And the relation among urinary, serum and intra-renal expression of miR-29a was highly consistent, and intra-renal miR-29a was closely related to kidney tissue injury. These results suggest that serum and urinary miR-29a level had the potential to be used as biological marker for kidney tissue injury of IgAN.
During the procession of renal fibrosis of IgAN, recent studies have found that TGF-β1/Smad3 signaling pathway is the main and final common pathway for renal fibrosis [31]. Previous studies demonstrate that TGF-β1/Smad3 signaling promotes renal fibrosis by inhibiting miR-29a [18]. Additionally, miR-29a could inhibit renal fibrosis by inhibiting TGF-β1/ Smad3 signaling [18]. Another study suggest that , in the cultured in vitro proximal tubular cells, primary mesangial cells, and podocytes, after exposed to TGF-β1, which targets the expression of collagen gene and up-regulates the expression of ECM proteins, reduced the expression of the miR-29a. For the resting cells and cells treated with TGF-β1, abnormal expression of miR-29 inhibited the expression levels of collagens I and IV mRNA and protein. Furthermore, they demonstrated a low level of miR-29 in rat models with early and advanced-stage renal fibrosis [19]. Another study found in Hepatocellular Carcinoma (HCC), miR-29a is able to mediate the EMT induced by TGF-β through influencing the DNA Methyltransferases (DNMT) [32]. This might be the specific pathogenesis among miR-29a with renal fibrosis.
The findings of current investigation indicated that the renal expression level of miR-29a of IgAN patients was considerably down-regulated than compared with those in the control group, which was in accordance with above previous findings.
Above results showed that the intra-renal expression of miR-29a decreased in patients with IgAN associated with TGF- β1/Smad3 mediated signal inhibit expression of miR-29a in the process of renal fibrosis, and elevated expression of miR-29a could also inhibit the TGF-β1/Smad3-signaling pathway, to achieve the purpose of the treatment of renal fibrosis. Available results suggested that miR-29a were renal-protective. As urinary, serum and intra-renal expression of miR-29a in IgAN patients was considerably down-regulated than compared with those in the control group, and their level was highly consistent. So urinary and serum expression of miR-29a have the potential as biological markers of renal fibrosis in IgA nephropathy.
In the present study, urinary and serum expression of miR-29a significantly inversely correlated with proteinuria and glomerulosclerosis, and urinary expression of miR-29a positively correlated with eGFR. These result highly suggest that urinary and serum decreased expression of miR-29a is closely related to the degree of renal damage in patients with IgAN, and urinary expression of miR-29a was closely related to the progress of renal function of IgAN. Urinary and serum expression of miR-29a could also be considered as an important indicator of degree of glomerularsclerosis in IgAN. Therefore, restoring miR-29a expression level might be an important treatment target of IgAN. Currently, research on the drugs effecting miR-29a expression is lacking. Therefore, finding a clinical available drug that can regulate the miR-29a expression can enhance the clinical efficacy for IgAN.
MMF is a potent immunosuppressant, elective inhibition of lymphocyte proliferation and antibody formation were regarded as the mechanism underlying the clinical efficacy. Previous study suggested that MMF could reduce proteinuria, and improve renal function in IgAN patients. Chen et al. [33] treated, either by MMF (1-1.5 g/d) or oral prednisone (0.8 mg/kg/d), 62 Chinese adult IgAN patients with advanced pathological lesions and proteinuria>2 g/d. One year after initiation of treatment, MMF-treated patients showed significant reduction of proteinuria and serum levels of cholesterol and triglyceride. A repeat renal biopsy performed in five patients after 9.8 ± 2.3 months of MMF treatment showed improvement of interstitial lesions. The authors concluded that MMF monotherapy for ≥ 12 months was superior to prednisone in reducing proteinuria in patients with an unfavorable histological grading. Previous study indicated that MMF could down-regulate the expression levels of MCP-1, ICAM-1 and TGF-β1 [21,22]. Regarding that miR-29a is crucial to the renal fibrosis and pathogenesis of IgAN, it hypothesized that miR-29a level may account for the function of MMF efficacy.
Nevertheless, the effect of MMF on miR-29a has been rarely performed. Considering that the process of renal fibrosis leads to decreased miR-29a. Hence, this study was designed to evaluate the influence of MMF on serum, urinary miR-29a expression.
MMF treatment could significantly increase the serum and urinary expression level of miR-29a, along with the decline of proteinuria. After MMF treatment, elevated expression of miR-29a could inhibit the TGF-β1/Smad3-signaling pathway, to inhibit the progress of renal fibrosis. We hypothesized that IgAN patients could restore the miR-29a expression after MMF administration. The serum, urinary and renal expression levels of miR-29a were highly consistent. So serum and urinary level of miR-29a is regarded as biological marker, which can assess the effect of MMF upon IgAN. But the specific mechanism needed further study.
Taken together, the present study found that the serum, urinary and renal expression of miR-29a were down-regulated of patients with IgAN. The urinary and serum miR-29a level is related with the severity of proteinuria and glomerularsclerosis, and urinary miR-29a level correlated with renal function. The results suggested urinary and serum expression of miR-29a might play a vital role in reflecting the pathogenesis and development of IgAN. As urinary, serum and intra-renal expression of miR-29s was highly consistent, so urinary and serum expression levels of miR-29a serve as biological markers of renal fibrosis in IgAN. MMF could significantly up-regulate the serum and urinary levels of miR-29a in IgAN. It may be a mechanism underlying the clinical efficacy of MMF in treating IgAN.
References
- Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol 2005; 16: 2088-2097.
- Berthoux FC, Mohey H, Afiani A: Natural history of primary IgA nephropathy. Semin Nephrol 2008; 28: 4-9.
- Tan CH, Loh PT, Yang WS. Mycophenolate mofetil in the treatment of IgA nephropathy: a systematic review. Singapore Med J 2008; 49: 780-785.
- Damico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis 2000; 36: 227-237.
- Cook HT. Interpretation of renal biopsies in IgA nephropathy. Contrib Nephrol 2007; 157: 44-49.
- Julian BA, Wyatt RJ, Matousovic K, Moldoveanu Z, Mestecky J. IgA nephropathy: a clinical overview. Contrib Nephrol 2007; 157: 19-26.
- Floege J. The pathogenesis of IgA nephropathy: what is new and how does it change therapeutic approaches? Am J Kidney Dis 2011; 58: 992-1004.
- Border WA, Noble NA. TGF-beta in kidney fibrosis: a target for gene therapy. Kidney Int 1997; 51: 1388-1396.
- Chihara Y, Ono H, Ishimitsu T, Ono Y, Ishikawa K, Rakugi H, Ogihara T, Matsuoka H. Roles of TGF-beta(1) and apoptosis in the progression of glomerulosclerosis in human IgA nephropathy. Clin Nephrol 2006; 65: 385-392.
- Del Prete D, Gambaro G, Lupo A, Anglani F, Brezzi B, Magistroni R, Graziotto R, Furci L, Modena F, Bernich P, Albertazzi A, DAngelo A, Maschio G. Precocious activation of genes of the renin-angiotensin system and the fibrogenic cascade in IgA glomerulonephritis. Kidney Int 2003; 64: 149-159.
- Lan HY, Chung ACK. Transforming growth factor-beta and Smads. Contrib Nephrol 2011; 170: 75-82.
- Wu W, Jiang XY, Zhang QL, Mo Y, Sun LZ, Chen SM. Expression and significance of TGF-beta1/Smad signaling pathway in children with IgA nephropathy. World J Pediatr 2009; 5: 211-215.
- Lee RC, Feinbaum RL, Ambros V. The C. elegansheterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75: 843-854.
- Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell 2006; 11: 441-450.
- Ruvkun G. The perfect storm of tiny RNAs. Nat Med 2008; 14: 1041-1045.
- Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev 2011; 91: 827-887.
- Lorenzen JM, Haller H, Thum T. MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol 2011; 7: 286-294.
- Wei Q, Arthur CKC, Xiao RH, Xiao MM, David SCH, Cheuk MY, Joseph JYS, Hui YL. TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 2011; 22: 1462-1474.
- Bo W, Radko K, Rosemarie C, Catherine EW, Bei X, Michal HE, Philip K, Merlin T, Karin JD, Paul G, Mark EC, Phillip K. Suppression of microRNA-29 expression by TGF-β1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol 2012; 23: 252-265.
- Utimura R, Fujihara CK, Mattar AL, Malheiros DM, Noronha IL, Zatz R. Mycophenolate mofetil prevents the development of glomerular injury in experimental diabetes. Kidney Int 2003; 63: 209-216.
- Wu YG, Lin H, Qi XM, Wu GZ, Qian H, Zhao M, Shen JJ, Lin ST. Prevention of early renal injury by mycophenolate mofetil and its mechanism in experimental diabetes. Int Immunopharmacol 2006; 6: 445-453.
- Wu Y, Dong J, Yuan L, Liang C, Ren K, Zhang W, Fang F, Shen J. Nephrin and podocin loss is prevented by mycophenolate mofetil in early experimental diabetic nephropathy. Cytokine 2008; 44: 85-91.
- Gang W, Bonnie CHK, Fernand MML, Kai MC, Philip KTL, Cheuk CS. Urinary miR-21, miR-29, and miR-93: novel biomarkers of fibrosis. Am J Nephrol 2012; 36: 412-418.
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461-470.
- Applied Biosystems. Endogenous controls for real-time quantitation of miRNA using TaqMan microRNA assays, application note TaqMan microRNA assays 2007.
- Moriyama T, Nakayama K, Iwasaki C, Ochi A, Tsuruta Y, Itabashi M, Tsukada M, Takei T, Uchida K, Nitta K. Severity of nephrotic IgA nephropathy according to the Oxford classification. Int Urol Nephrol 2012; 44: 1177-1184.
- Rifai N, Gillette MA, Carr SA. Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat Biotechnol 2006; 24: 971-983.
- Xi Y, Nakajima G, Gavin E, Morris CG, Kudo K, Hayashi K, Ju J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA 2007; 13: 1668-1674.
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, OBriant KC, Allen A. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008; 105: 10513-10518.
- Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA 2005; 11: 1753-1761.
- Wang W, Koka V, Lan HY. Transforming growth factor-beta and Smad signalling in kidney diseases. Nephrology 2005; 10: 48-56.
- Takayuki K, Yasuteru K, Eiji K, Masashi N, Osamu K, Tooru S. Involvement of miRNA-29a in epigenetic regulation of transforming growth factor-ß-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Hepatology Res 2014; 44: 907-919.
- Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang Y, Liu S, Tang L. A randomized control trial of mycophenolate mofeil treatment in severe IgA nephropathy. Zhonghua Yi Xue Za Zhi 2002; 82: 796-801.