- Biomedical Research (2006) Volume 17, Issue 2
Short communication: Amino acid profiles among colorectal cancer patients
Abdulbari Bener1,2, Muzeyyen Dogan3, Ismail Abou Azab4, Ali Rashed5 and Midhat Siddiqui61Department of Medical Statistics & Epidemiology, Hamad General Hospital, Hamad Medical Corporation Doha, State of Qatar
2Department Evidence for Population Health Unit, School of Epidemiology and Health Sciences, The University of Manchester, Manchester, United Kingdom
3Department of Chemistry, Faculty of Sciences, Yildiz Technical University, Istanbul, Turkey
4Department of Surgery, Al-Ain Hospital, MOH, Al-Ain, United Arab Emirates
5Department of Oncology, Tawam Hospital, GAHS, Al-Ain, United Arab Emirates
6Department of Surgery, Queen Mary Hospital, NHS, London, United Kingdom
- *Corresponding Author:
- Abdulbari Bener
Department of of Epidemiology and Medical Statistics
Hamad Medical Corporation Hamad General Hospital
Weill Cornell Medical College in Qatar PO Box 3050
Doha State of Qatar
Tel: + 974-439 3765
Fax: + 974-439 3769
E-mail: abener@hmc.org.qa, abaribener@hotmail.com
Accepted date: May 24 2006
Abstract
Epidemiology. Case-control. Colorectal cancer. Amino acid. UAE
Keywords
Epidemiology. Case-control. Colorectal cancer. Amino acid. UAE
Introduction
Colorectal cancer is one of the most common human ma-lignancies, and has been well documented [1]. Carcinoma of the colon and rectum accounts for more than 150,000 deaths annually worldwide. It is considered the second most frequent cause of death in most of the Western Countries after lung cancer in men and breast and lung cancer in women [2]. Microsatellite instability (MSI) is one form of genomic instability that occurs in 10% to 20% of sporadic colon tumors and almost all of the he-reditary non-polyposis colon cancers [3]. However, little is known about how environmental factors (e.g., diet) may influence MSI in sporadic colon cancer. Colon cancer is common in women and rectal cancer is common in men [4,5] Furthermore, some study showed that colorec-tal cancer is the leading type of cancer in Western coun-tries. Over the last decades, the trend of colorectal cancer has been generally more favorable for women than men [6]. New studies in molecular biology and genetics of colorectal cancer have provided clues to its etiology, and clues for the identification of the high risk populations who are most likely to benefit from preventive measures [5].
Amino acids are the building blocks of proteins. They are vital to the construct and integrity of every cell in the body: without them the cells would first fail to hold their morphology, and then fail to perform their unique metabolic functions [7]. Arginine is an essential amino acid for tumour cells. It is helpful to improve the life quality if we take patients’ nutrition into account according to the characteristic amino acid metabolism before treating the cancer patients. Hence, it is important to find the amino acid profile in cancer patients.
However, since colorectal cancer is highly influenced by environmental changes including life style factors [3,4,8-11], it is obvious that in the coming years; colorectal cancer will be one of the major types of cancer to be tackled in the Emirates. The aim of this study is to determine the amino acid profile among the colorectal cancer patients.
Subject and Methods
This study is based on the data of 108 colorectal cancer patients who were referred to Tawam, Al-Ain, and Al-Saif Hospitals in the United Arab Emirates and underwent surgeries for colorectal carcinoma during the period from 1998 to 2003. There were 74 males and 34 females with a male/female ratio of 2.2:1. Their ages ranged from 18 to 65 years with a mean and a standard deviation of 47.2 ± 9.7 (median age 47 years).
The clinicopathologic and histological data
The patients’ files in the surgery department were reviewed to obtain the following operative data: age, gender, resection procedure, tumor size, tumor stage, depth of invasion, type of operation (whether emergency or elec-tive), type of resection (whether radical or palliative), lymph node involvement, intraperitoneal tumor spread and presence of liver metastasis, nodal involvement, dif-ferentiation grade, and p53 expression.
Immunohistologic staining
We have used monoclonal antibody (MAb) against p53 protein, Pab1801 (Oncogene Science Inc., Manhasset, NY), we performed the avidin-biotin complex immunoperixidase method to detect bound p53 Mab [8-12]. After deparaffinization and elimination of endogenous peroxid-ase activity, p53 MAb was incubated overnight at room temperature. After being washed in phoshate-buffed sa-line solution, sections were incubated with biotinylated horse antimouse immunoglobulin G antibody and seubse quentlywith avidin-biotin-linked peroxidase. The substrate used for detection of p53 MAb binding was 0.05% 3,3’ -diaminobenzidine with 0.01% hydrogen peroxide. Sections were scored as positive if a distinct nuclear immuno-recation for p53 was found in the identifiable tumor cells.
Detection of Amino Acid
A standard amount of 0.8 ml of venous blood was collected in a lithium heparinised bottle and was centrifuged immediately for five minutes at 2000 rpm [13]. From this sample, 2000μl of plasma was deproteinised by adding 20μl of 50% sulphosalicylic acid and was then centrifu-ged at 10,000 rpm for 10 minutes at 4°C. The supernatant was stored at -70°C until analysis at which time it was diluted at 1:1 with a Li-s Beckman buffer along with the international standard. An analysis with the Ion Exchange Chromatography was carried out for individual free amino acids according to Spackman et al [14,15] using a Beckman 6300 automatic amino acid analyzer by the Beckman method. A three Li-buffer system was used in a 10cm high (and 0.4 cm diameter) cation-exchange resin column at a pressure of around 1200 psi and at 20ml/h buffer flow. The method is based on the post column derivatisation by ninhydrin colouring reagent at 135°C. The derivatised amino acids were detected at 570 nm, while proline and hydroxyprooline at 440nm.
Helicobacter Pylori Serology
The test was performed according to the manufacturer’s instructions. IgG antibodies against a low molecular weight fraction of H. pylori antigens were measured in duplicate with a validated in house indirect enzyme-lin-ked immunosorbent assay [ELISA]. The result was considered positive if IgG and IgA anti-H. pylori anti-body titers were > 300 and > 250 respectively. The sensi-tivity values for IgG and IgA H .pylori serology tests were 74% and 51% respectively (p<0.001).
Fisher exact and Chi-square tests were used to ascertain the association between two or more categorical variables. Student-t and non-parametric Mann Whitney test were used to ascertain the significance of differences between mean values of two continuous variables. The level p<=0.05 was considered as the cut-off value for signifi-cance.
Results
During the last 6 years, a total of 108 patients with colo-rectal cancer were diagnosed in the United Arab Emirates.
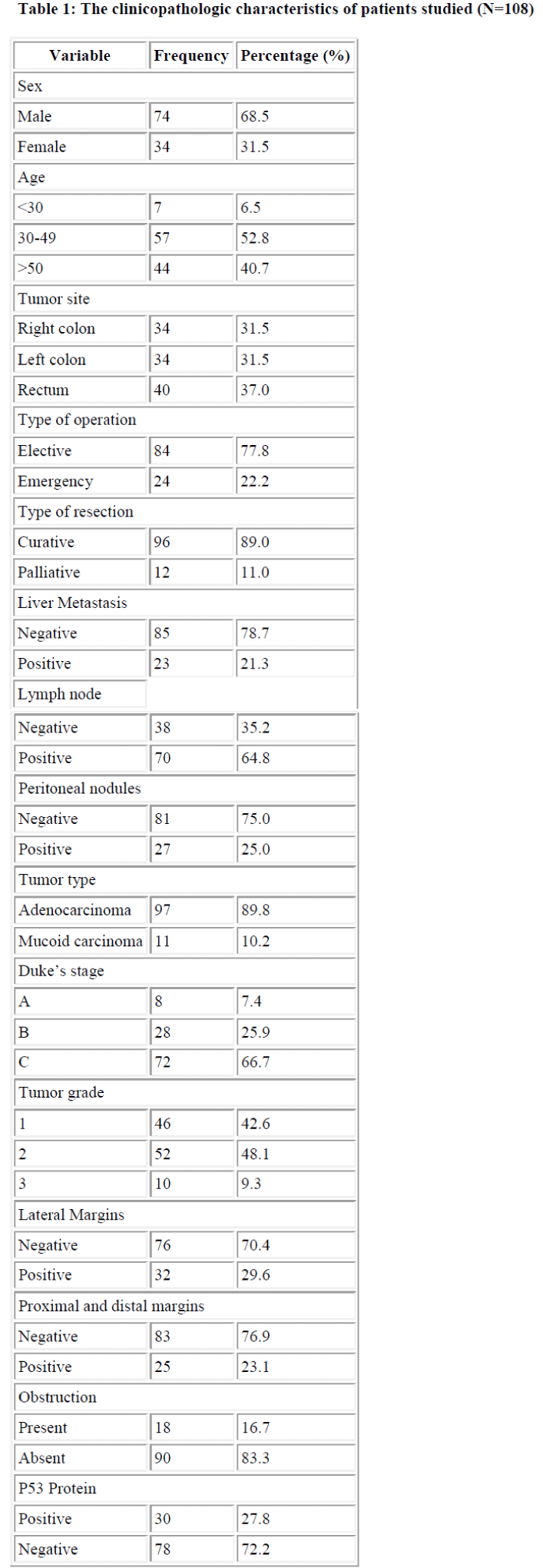
The socio-demographic and clinicopathologic characteris-tics of the studied patients are shown in Table 1. There were 74 males (68.5%) and 34 females (31.5%). The mean age at the time of diagnosis was 47.2±9.7 years with the median age of 47 years. The age of highest occurrence was between 41-50 years and accounted to 34.2%, while the lowest accounted to 7.9% of the patients and were below the age of 30 years. The mean overall survival time was 63.7 ± 7.7 months with a range of 6 and 120 months.
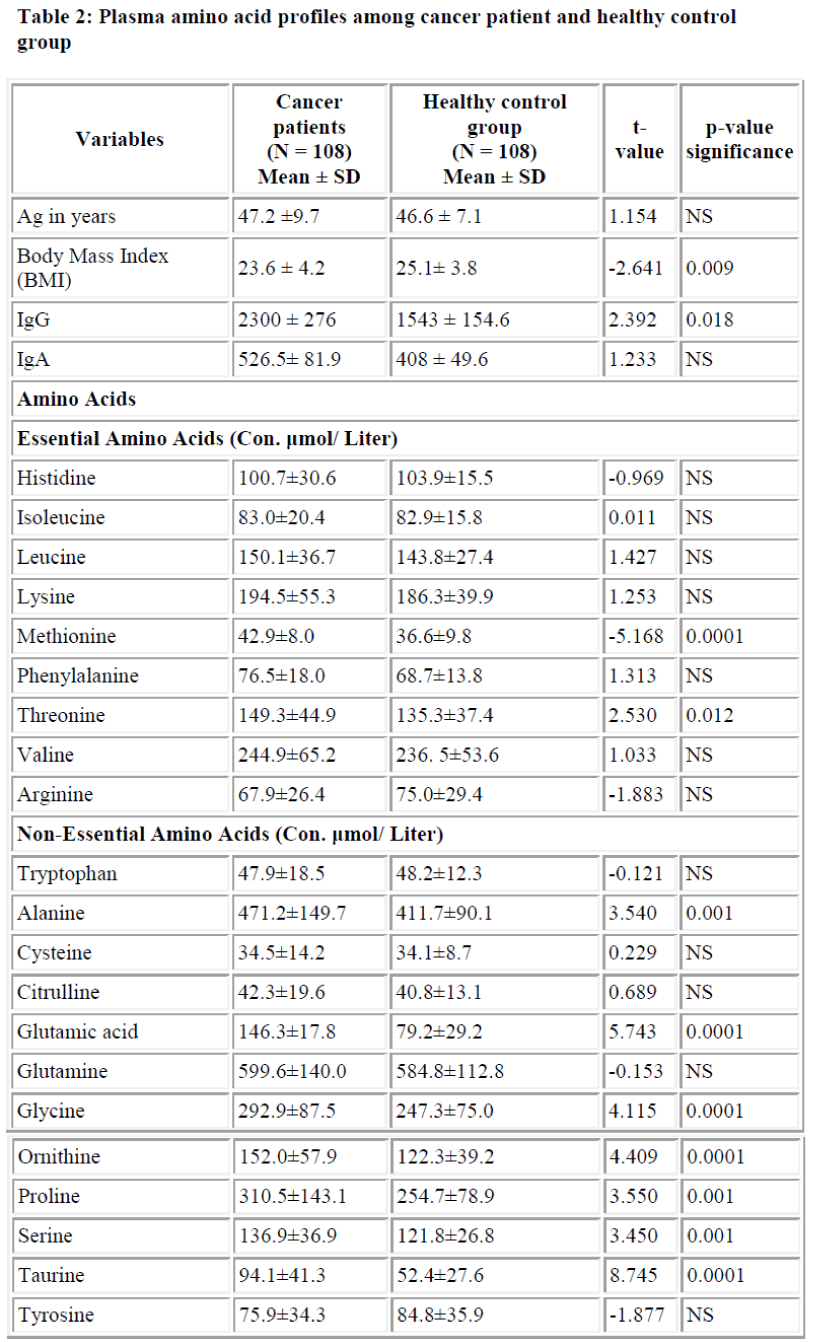
Table 2 shows plasma amino acid profiles among the co-lorectal cancer patients and among the healthy control group. H. pylori serology was determined in all subjects. As can be seen from this table the difference between cancer patients and healthy control group concerning IgG was statistically significant (p<0.018). Also, the amino acid analysis showed higher mean values among the colo-rectal cancer patients than the control group for all esse-tial amino acids except for Arginine and Histidine and most of them were not significant except threonine (p<0.012), and methionine (p<0.0001). Ornithine, taurine, glutamic acid, serine, glycine, proline and alanine as non-essential amino acids showed significantly higher values for the cancer patients when compared with the healthy control group and most of them were highly significant (p<0.0001).
Discussion
Cancer of colon and rectum is the third most frequent type of cancer worldwide in males and females [1]. The most recent results for the most common cancers in terms of new cases ranked lung cancer first (1.2 Million), breast cancer second (1.05 Million) followed by colorectal cancer (945,000) [1]. In European Countries [10], the most common primary sites were lung (22% of all cancers), followed by colon and rectum (12%) for men and for females breast cancer ranked first (26%) then colon and rectum (14%).
The age standardized incidence rate for colorectal cancer in United Arab Emirates was 3.1/ 100,000 person per year. There must be an important protective factors, pos-sibly dietary, that can contribute to lowering this risk.
Therefore, this would be an important area of research for future studies. Some epidemiological studies demonstrated the importance of genetic and environmental fac-tors which play an important role in the pathogenesis of carcinoma of the colon and rectum [4,11,12]. Worldwide, the incidence of colorectal cancer varies widely, it varies from 1.7 cases per 100,000 person per year in some Western African countries to 51.7 cases per 100,000 per-son per year in North America [1]. The incidence rate of colorectal cancer in the Arabian and the Gulf Countries showed 3.8/100,000 in Jordan [17], 7.5/100,000 in Qatar [18], and 4.5/100,000 in Saudi Arabia [11-20]. These rates are in agreement with our results.
Our results showed that younger patients have an earlier recurrence and have poorer survival when compared to older patients. These data are in agreement with several studies [5,13,14]. The age of highest incidence was above 40 years, which is similar to the findings of other reports of Saudi Arabia [10]. United Arab Emirates [6], Jordan [17], Qatar [18], UK [1], USA [1], Pakistan [19], and Af-rica [1].
Recently, some studies showed that colorectal cancer is the leading cancer in Western countries. During the last decade, its trends have been generally more favorable for women than men [5] and our results are not in consistent with these Western results. However in European countries, its trends took a different pattern during the last decades which was more favorable for men than women [10]. The profile varies greatly in different populations, and the evidence suggests that this variation is mainly a conse-quence of different lifestyle and environmental factors, which should be amendable to preventive interventions [1].
Most of the researchers have found that there is a direct correlation between the fat intake in the diet and the incidence of colon cancer [12]. The low incidence of colorectal carcinoma in most developing countries have been attributed to the fiber-rich diet consumed in these countries [11]. It has been suggested that fiber-rich diet gives protection against colonic carcinogenesis [3].
A study conducted by the National Cheng Kung University Hospital College of Medicine, Taiwan on Plasma amino acid levels in patients with colorectal cancers and liver cirrhosis with hepatocellular carcinoma revealed that about one third of early or late colorectal cancer patients had body weight loss more than 10% in half a year and were defined as malnourished. For individual amino acids, in early colorectal cancer patients, the plasma level of most essential amino acids and non-essential amino acids decreased significantly. In the last stage of colorectal cancer patients, plasma levels of most essential and non-essential amino acids decreased more obviously. The difference in concentration was also noticeably significant Our study demonstrated that all the essential amino concentration in serum were higher, but Histidine and Arginine were decreased in colon cancer patients, of which the non-essential amino acids, most of them were elevated in serum except Tryptophan and Tyrosine when compared to healthy control group. A similar study finding was reported in a study conducted by Wang LB et al [22] that the serum concentration of most essential amino acids (Leucine, Isoleucine, Phenylalanine, threonine, lysine) and some other non essential amino acids (tyrosine, Proline, glutamine) in cancer tissues were obviously higher than those in normal tissue [22]. This suggests that colorectal cancer patients might need more of these amino acids.
A remarkable difference in amino acid profiles between the colorectal cancer patients and the control subjects is found in this study and most of the differences are statistically significant in respect of non-essential amino acids. The difference in essential amino acids concentration was noticeably insignificant except for Methionine and Theronine. Furthermore, some studies showed that the concentration of amino acids in subjects might be correlated with the some diseases [23].
In general, the incidence rate of colorectal carcinoma in United Arab Emirates is similar to that reported from other neighboring Arabian and Gulf Countries like Jordan [17], Saudi Arabia [11,20], and Qatar [18]. The main reasons of low incidence of colorectal cancer in these coun-tries could be due to the dietary factors which is intake of fruits and vegetables. However, colorectal cancer in these countries tends to affect the middle aged group in a higher percentage than other parts of the world.
In conclusion, the results revealed that the plasma amino acid concentrations were higher among colorectal cancer patients than among the control subjects. There was statistically a significant relationship between patients and controls in respect of IgG. A comprehensive cancer health education and screening program should be well planned and should be implemented for an early detection and prevention of this disease. We hope that future prospective studies will help us to identify the genetics and the environmental factors associated with the changing epidemiological pattern of colorectal cancer in the United Arab Emirates.
Acknowledgements
We would like to thank Dr. Taleb M. Al-Mansoor for the blood samples and data collection. We are grateful to Ms. Fatima R. M. Al-Neamy for the amino acid analysis and Mr. Asad Usmani for the H. Pylori Serology analysis.
References
- Parkin DM. Global cancer statistics in the Year 2000. Lancet Oncol 2001; 2: 533-543.
- Ahmed FE. Colon cancer: prevalence, screening, gene expression and mutation, and risk factors and assessment. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2003; 21: 65-131.
- Satia JA, Keku T, Galanko JA, Martin C, Doctolero RT, Tajima A, Sandler RS, Carethers JM. Diet, life-style, and genomic instability in the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev. 2005; 14: 429-436.
- Al-Shamsi SRK, Bener A, Al-Sharhan M, Al-Mansor TM, Abou Azab I, Rashed A, Kakil IR, Amiri KMA. Clinicopathological Pattern of Colorectal Cancer in the United Arab Emirates. Saudi Med J 2003, 24: 518-522.
- Buyse M, Thirion P, Carlson RW, Burzykowski T, Molenberghs G, Piedbois P. Relationship between tumor response to first line chemotherapy and survival in advanced colorectal cancer: a meta analysis. Lancet 2000; 356: 373-378.
- Fernandez E, Bosetti C, La Vecchia C, Levi F, Fioretti F, Negri E. Sex differences in colorectal cancer mortality in Europe, 1955-1996. Eur J Cancer Prev, 2000 9: 99-104.
- www.mgwater.com/taurine.shtml (accessed on 18/5/ 2006)
- El-Ghazawy IMH, Bener A, Saad Eldin S, Abou Azab I, Siddiqui M. Predictors of outcome of surgical treatment for colorectal carcinoma in the United Arab Emirates. WHO Bulletin Eastern Medit Health J, 2001; 7: 1-9.
- Yeh CC, Hsieh LL, Tang R, Chang-Chieh CR, Sung FC. MS-920:DNA repair gene polymorphisms, diet and colorectal cancer risk in Taiwan. Cancer Lett. 2005;224: 279-88.
- Bray F, Sankila R, Ferlay J, Parkin DM. Estimates of cancers in incidence and mortality in Europe in1999. Eur J Cancer, 2002, 38: 99-166.
- Mansoor I, Zahrani I, Abdul Aziz S. Colorectal cancers in Saudi Arabia. Saudi Med J, 2002, 23: 322-327.
- Motojima K, Furui J, Kohara N, Ito T, Kanematsu T. Expression of p53 protein in gastric carcinomas is not independently prognostic. Surgery. 1994; 116: 890-5.
- Bener A, Lestringant GG, Dogan M, Ibrahim A, Al-shadli, Pasha MAH, Islam MR (1998) Respiratory Symptoms and skin disorders in sewage workers. J En-vironment Sci & Health Part A, 33A: 1657-1674.
- Spackman DH, Stein WH, Moore S. Automatic recording apparatus for use in the chromatography of amino acids. Anal Chem;1958; 30: 1190-9.
- Beshwari MMM, Bener A, Almehi AM , Ameen A, Ouda HZ, Ibrahim A, Pasha MAH. Amino acid profiles in farm workers. J Environment Int, 1999; 25: 411-416.
- Dent JC , McNultt CAM, Uff JS, Gear MW, Wilkinson SP. Campylobacter pylori urease: A new serological test. Lancet 1988; I:1002.
- Al-Jaberi TM, Ammari F, Gharieybeh K, Khammash M, Yaghan RJ, Heis H et al Colorectal adenocarcinoma in a defined Jordan population from 1990 to 1995. Dis Colon Rectum; 1997; 40: 1089-94.
- Kakil IR, Awidi AS, Mubarak AA, Al-Homsi UM. Study of colorectal cancer in Qatar. Saudi Med J, 2001, 22: 705–707.
- Bhurgri Y, Bhurgri A, Hassan SH, Zaidi SHM, Rahim A, Sankaranarayanan, Parkin DM. Cancer incidence in Karachi, Pakistan: First results from Karachi Cancer Registry. Int J Cancer; 2000, 85: 325-329.
- Ayyub MI, Al-Radi AO, Khazeindar AM, Nagi AH, Maniyar IA. Clinicopathological trends in colorectal cancer in a tertiary care hospital. Saudi Med J, 2002, 23: 160-163.
- Lee JC, Chen MJ, Chang CH, Tiai YF, Lin PW, Lai HS, Wang ST. Plasma amino acid levels in patients with colorectal cancers and liver cirrhosis with hepato-cellular carcinoma. Hepatogastroenterology 2003; 50: 1269-1273.
- Wang LB, Zhang SZ, Ding KF, Zheng S. A study of the free amino acids uptake in Colon Cancer. Zhejiang Y. 1997; 19: 208-209.
- VanderJagt DJ, Kanellis GJ, Isichei C, Pastuszyn A, Glew RH. Serum and urinary Amino acid levels in sickle cell disease. J Trop Pediatrics, 1997; 43:220-225.